Access Agilent eNewsletter, October 2013
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Analysis of N-glycans derived from monoclonal antibody by capillary electrophoresis-electrospray ionization mass spectrometry
By Suresh Babu CV
Agilent Application Scientist
Glycosylation is one of the most important post-translational modifications involving attachment of glycan moieties to proteins. Variable glycosylation on monoclonal antibodies (mAb) can have impacts on biological activity and immunogenicity. Due to the importance of mAb as therapeutic agents, there is a growing demand for monitoring the carbohydrate structures attached to mAb.
Until now, various widely used analytical techniques for profiling mAb N-glycans have faced significant challenges in detection of low levels of glycan species. Recently, capillary electrophoresis (CE) has gained much attention in analyzing the glycoproteins, delivering high efficiency separations in shorter run times. Further, there is growing interest in coupling CE to mass spectrometry (MS) for higher sensitivity and better compound identification with accurate mass measurements.
The application described below demonstrates the analysis of N-linked glycan of recombinant monoclonal antibody glycans (mAb) by employing a CE/MS platform. The method involves enzymatic cleavage of glycans from mAb by PNGase F, followed by fluorescent 8-aminopyrene-1,3, 6-trisulfonic acid trisodium salts (APTS) labeling of glycans. The APTS-labeled glycans were purified and analyzed by using CE/MS.
Establishing the experimental protocol
Experiments were conducted using an Agilent 7100 Capillary Electrophoresis system coupled to an Agilent 6520 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) with the following modules:
- Agilent 7100 Capillary Electrophoresis system
- Agilent CE/MS Adapter Kit
- Agilent CE-ESI-MS Sprayer Kit
- Agilent 1200 Series Isocratic HPLC Pump (automated sheath liquid delivery)
- Agilent 6520 Accurate-Mass Q-TOF LC/MS with Dual Electrospray Source
- Agilent MassHunter software (Bioconfirm)
The Agilent CE system (Figure 1) was coupled to an Agilent 6520 Q-TOF LC/MS system equipped with dual electrospray source and orthogonal coaxial sheath liquid interface. The sheath liquid was delivered by an Isocratic Pump equipped with a 1:100 flow splitter. The sheath liquid mainly served to connect the CE outlet electrically to ground potential at the sprayer, and ensured that voltage was constantly applied across the length of the separation capillary for stable electrospray at constant flow rates.
 Enlarge
Enlarge
Figure 1. Agilent Capillary Electrophoresis & CE/MS system.
 Enlarge
Enlarge
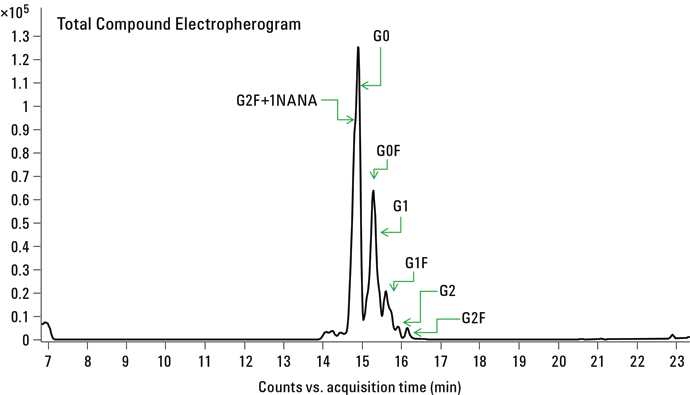
Figure 2. CE/MS of APTS labeled N-glycans released from mAb.
 Enlarge
Enlarge
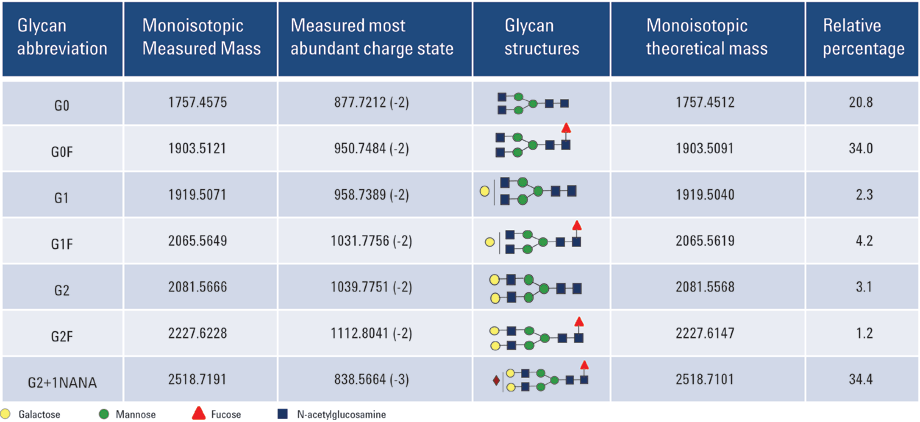
Table 1. mAb N-glycans identified using CE/MS.
Identifying N-linked glycans in the mAb
Figure 2 shows the CE/MS total compound profile for N-linked glycans released from the recombinant mAb. Using a PVA-coated capillary, all the mAb glycans were migrated within a 20 minute separation time. The uncharged N-linked glycan species G0, G0F, G1, G1F, G2, and G2F were successfully identified in replicate runs. In addition, mono-sialylated glycan moiety G2F+1NANA was also observed. All the peak assignments were based on accurate mass measurements from Q-TOF analysis. Table 1 summarizes the glycans identified in this study.
 Enlarge
Enlarge
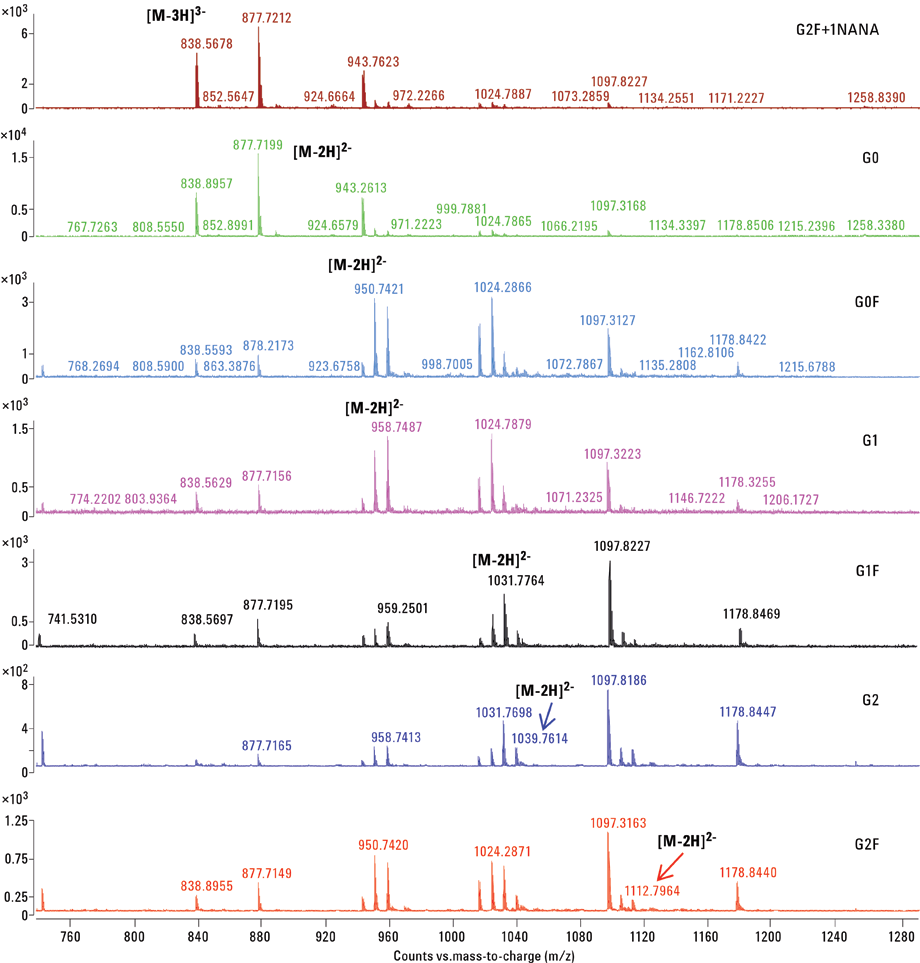
Figure 3. CE/MS mass spectra of APTS labeled mAb N-glycans.
Documenting the mass spectrum and relative quantification of N-linked glycans
Estimating the absolute/relative glycan quantity levels is of paramount importance in the therapeutic product development pipeline. Therapeutic products are sensitive to degradation and any minimal modifications.
The mass spectrum for each of the individually resolved glycans in this experiment is depicted in Figure 3. The most abundant charge-states observed in the mass spectrum are indicated. The percentage volume relative to total compound peaks volume was estimated and relative quantifications of mAb-released glycans are shown in Table 1. In the present case, the major form of the glycoform was found to be G2+1NANA.
These results clearly indicate that the Agilent CE/MS System can be used effectively as an alternative analytical tool for monitoring glycan profiles. CE/MS coupled separations avoid contaminations, due to minimal sample handling steps, and the buffers used for CE separations are highly compatible with the electrospray ionization. The current approach is sensitive and accurate in glycan profiling as compared to conventional CE-LIF.
Dig deeper
To learn more about this experimental protocol and for a deeper look at our results, download the complete Agilent Application Note 5991-1020EN today.
>> Update My Profile | Subscribe to Access Agilent | Article Directory