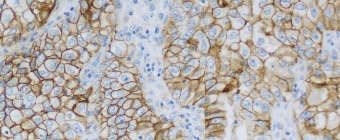
PD-L1 IHC 22C3 pharmDx Testing for NSCLC

- KEYTRUDA is the first and only anti-PD-1 monotherapy FDA-approved to treat metastatic NSCLC at first line—see the KEYTRUDA product label for prescribing information details
- Tumor PD-L1 overexpression is a biomarker for response to anti-PD-1 therapy—in a KEYTRUDA clinical trial, 57% of patients with NSCLC had tumors that exhibited PD-L1 expression
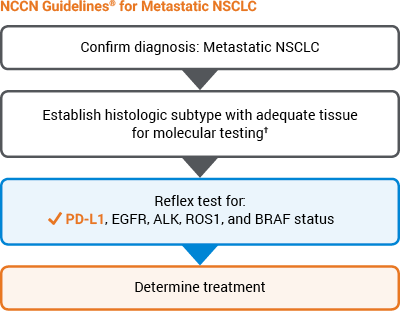
- IHC testing for PD-L1 expression enables identification of patients most likely to benefit from PD-1 checkpoint inhibitors
- Pembrolizumab (KEYTRUDA) is a category 1* recommendation for metastatic NSCLC patients who express ≥ 50% PD-L1—the highest National Comprehensive Cancer Network® (NCCN®) category of evidence and consensus
- In first-line treatment: Recommended for patients with PD-L1 expression levels ≥ 50% when test results for sensitizing EGFR mutations, ALK rearrangements, ROS1 rearrangements, and BRAF V600E mutations are negative or unknown
- In subsequent therapy: Recommended as a treatment option for patients with PD-L1 expression levels ≥ 1%, if pembrolizumab not previously given
* Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
† Consider rebiopsy if appropriate. If repeat biopsy is not feasible, plasma testing should be considered.

Proven in KEYNOTE-024 and KEYNOTE-010 for PD-L1 results in NSCLC that you can rely on
- PD-L1 IHC 22C3 pharmDx was used to assess PD-L1 expression and select patients for KEYTRUDA therapy in the KEYNOTE-024 and KEYNOTE-010* trials
- Results from the KEYNOTE-024 and KEYNOTE-010 trials, including the clinical benefit of testing for PD-L1 expression, are available at https://www.keytruda.com/hcp/nsclc/
* The clinical trial assay (CTA) version of PD-L1 IHC 22C3 pharmDx was used.
Table 1: PD-L1 prevalencea in patients with NSCLCb screened for KEYNOTE-024c

a. Merck & Co., data on file; b. Patients screened for enrollment in KEYNOTE-024 NSCLC; c. International phase 3 study comparing pembrolizumab with investigator's choice platinum-containing chemotherapy (including pemetrexed+carboplatin, pemetrexed+cisplatin, gemcitabine+cisplatin, gemcitabine+carboplatin, or paclitaxel+carboplatin) in patients with non-small cell lung carcinoma who were previously untreated for advanced metastatic disease. ClinicalTrials.gov number NCT02142738.
Table 2: PD-L1 prevalenced in patients with NSCLCe screened for KEYNOTE-010f

d. Merck & Co., data on file; e. Patients screened for enrollment in KEYNOTE-010 NSCLC; f. International phase 2/3 study comparing pembrolizumab with docetaxel in patients with non-small cell lung carcinoma who have experienced disease progression after platinum-containing system therapy. ClinicalTrials.gov number NCT01905657.
Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) and urothelial carcinoma tissues using EnVision FLEX visualization system on Autostainer Link 48.
Non-Small Cell Lung Cancer (NSCLC) PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. The specimen should be considered to have PD-L1 expression if TPS ≥ 1% and high PD-L1 expression if TPS ≥ 50%.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for expression cutoff values guiding therapy in specific clinical circumstances.
Urothelial Carcinoma PD-L1 protein expression in urothelial carcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. The specimen should be considered to have PD-L1 expression if CPS ≥ 10.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying urothelial carcinoma patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for specific clinical circumstances guiding PD-L1 testing.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2018. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2018. 3. European Commission approves KEYTRUDA® (pembrolizumab) for first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression with no EGFR or ALK positive tumor mutations [news release]. Kenilworth, NJ; Merck & Co., Inc.; January 31, 2017. http://www.mrknewsroom.com/news-release/oncology-newsroom/european-commission-approves-keytruda-pembrolizumab-first-line-treatm. Accessed December 21, 2017. 4. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. 5. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. 6. Reck M, Rodríguez-abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. 7. Data on file. Merck & Co., Inc. 8. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.1.2018. © National Comprehensive Cancer Network, Inc. 2017. All rights reserved. Accessed December 21, 2017. To view the most recent and complete version of the guideline, go online to www.NCCN.org. National Comprehensive Cancer Network® (NCCN®) makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.