Intended Use
For in vitro diagnostic use
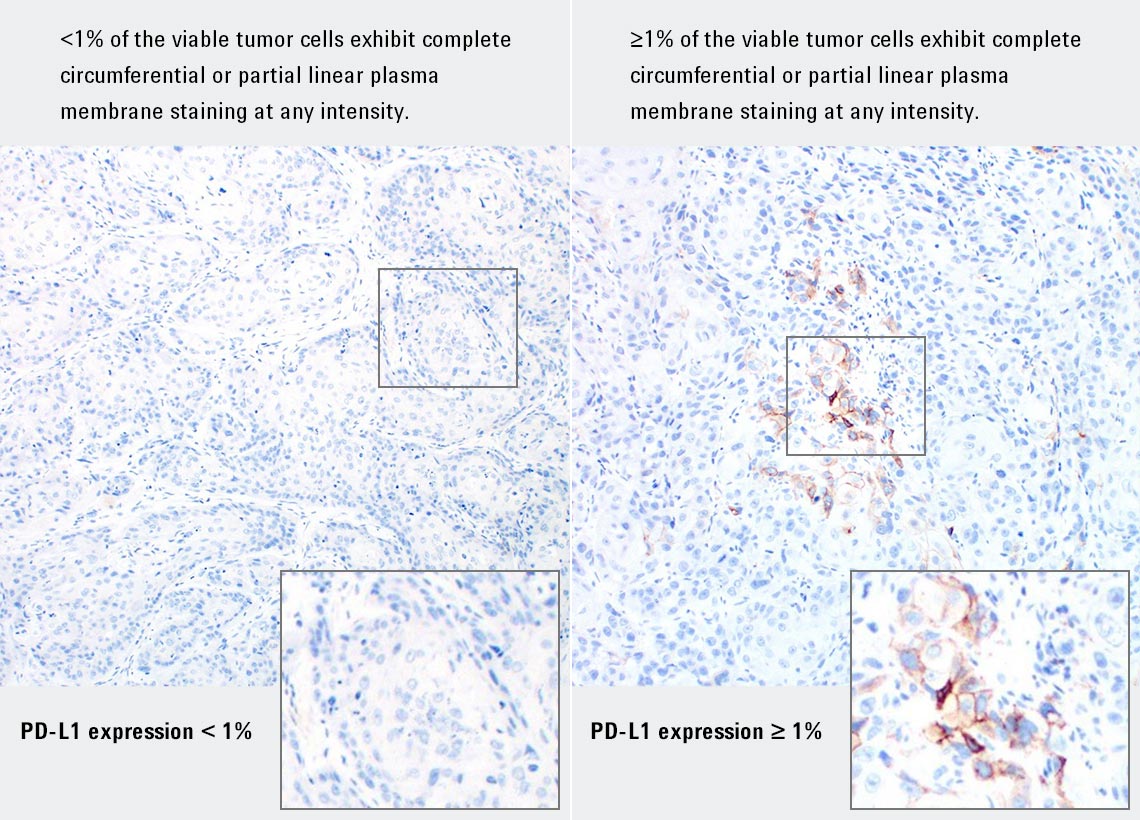
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-squamous non-small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and melanoma tissues using EnVision FLEX visualization system on Autostainer Link 48. PD-L1 protein expression is defined as the percentage of evaluable tumor cells exhibiting partial or complete membrane staining at any intensity, as defined by the specific tumor indication staining interpretation guidelines in the instructions for use (IFU).
PD-L1 expression as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC and SCCHN may be associated with enhanced survival from OPDIVO® (nivolumab).
PD-L1 expression as detected by PD-L1 IHC 28-8 pharmDx in melanoma may be used as an aid in the assessment of patients for whom OPDIVO® (nivolumab) and YERVOY® (ipilimumab) combination treatment is being considered.