Developmental Biology: Zebrafish Applications
July 2021
The zebrafish model system has seen a rapid expansion in usage over the past two decades for both translational and basic research paradigms. This growth in zebrafish popularity has been driven by a powerful combination of compelling practical advantages and an impressive repertoire of research tools. One of the most valued attributes of zebrafish is the optical transparency of young fish, providing unparalleled access to a whole vertebrate organism using non-invasive microscopy techniques. Accordingly, fluorescence microscopy-based phenotypic analysis has become mainstay in zebrafish-based research workflows.
In this TekTalk edition, we have compiled resources that provide guidance and tips for fluorescence-based zebrafish applications, including both image-based and reader-based investigations.
Featured Applications
Quantitative Microscopy of Angiogenesis in Zebrafish Embryos
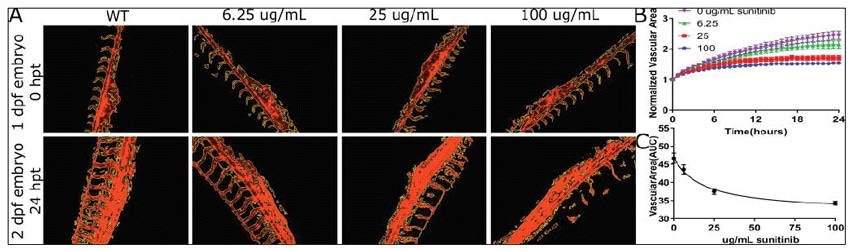
Controlled angiogenesis is essential for proper embryonic development and has implications for treatment of conditions such as cancer and heart disease. Zebrafish provide an opportunity to examine angiogenesis in vivo through the development of transgenic zebrafish expressing fluorescent markers of the vascular epithelia. Here we demonstrate how in vivo time-lapse fluorescence imaging can quantitatively evaluate vascularization in zebrafish transgenic embryos treated with angiogenesis inhibitors.
Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Cytation C10 Confocal Imaging Reader
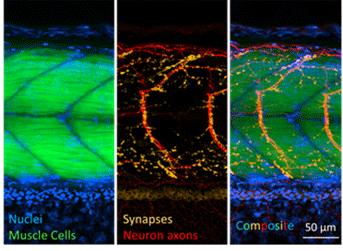
Zebrafish present a unique opportunity for microscopy-based investigation of motor neurons and musculature in vivo in an intact vertebrate organism. Fluorescence microscopy is a powerful tool in the evaluation of the nervous system and neurodegenerative disease phenotypes. Limitations to widefield fluorescence imaging, however, can complicate analysis of single cells deep in tissue. Confocal imaging overcomes limitations of widefield imaging deep in zebrafish, as shown in this application bulletin examining spinal motor neuron and muscle units in embryonic zebrafish.
Product Spotlight
Lionheart FX Automated Microscope
Lionheart FX Automated Microscope offers up to 100x magnification with brightfield, color brightfield, phase contrast and fluorescence channels for fixed and live cell applications. Optional incubation to 40°C, CO2/O2 control, and a humidity chamber optimize live cell assays. Gen5 software makes cell image capture, processing, analysis, and publication-ready data and images quick and effortless.
Cytation C10 Confocal Imaging Reader
Cytation C10 Confocal Imaging Reader combines automated confocal and widefield microscopy with conventional multi-mode microplate reading. The spinning disk confocal module adds increased resolution and optical sectioning capabilities to the Cytation range. Cytation C10 also includes widefield fluorescence, brightfield and phase contrast optics. The multi-mode module has variable bandwidth monochromator-based optics for specificity and sensitivity. System control, image and data analysis provided by BioTek's Gen5 software.
Tek Tips
Explore whether widefield or confocal imaging best suits your microscopy needs
The Cytation C10 confocal imaging reader makes capturing both widefield and confocal fluorescence images simple and automated. Confocal imaging excels at eliminating light originating outside of the focal plane, but it is important to recognize that not all samples benefit from this reduction for analysis. In the example shown in Figure 1A, this evaluation required capturing a large section of the vascular structure of the zebrafish tail into a single image. This transgenic zebrafish line expresses fluorescence only in the region of interest (vasculature), with essentially no background signal. Here, the confocal modality is not advantageous over widefield for this purpose: a single image using lower magnification and widefield optics rapidly produces the high-quality images necessary for analysis Depending on your sample characteristics, widefield imaging could potentially result in shorter image acquisition times.
On the other hand, the sample in Figure 1B clearly benefits from imaging with the confocal modality. Here the experiment calls for identification of a single motor neuron deep in the zebrafish tail, and the immunostaining protocol used generates staining in other neuron types above and below the focal plane of the cell of interest. This out of focus light in the widefield images interferes with the segmentation of the motor neuron, whereas the confocal modality achieves proper identification of the motor neurons.
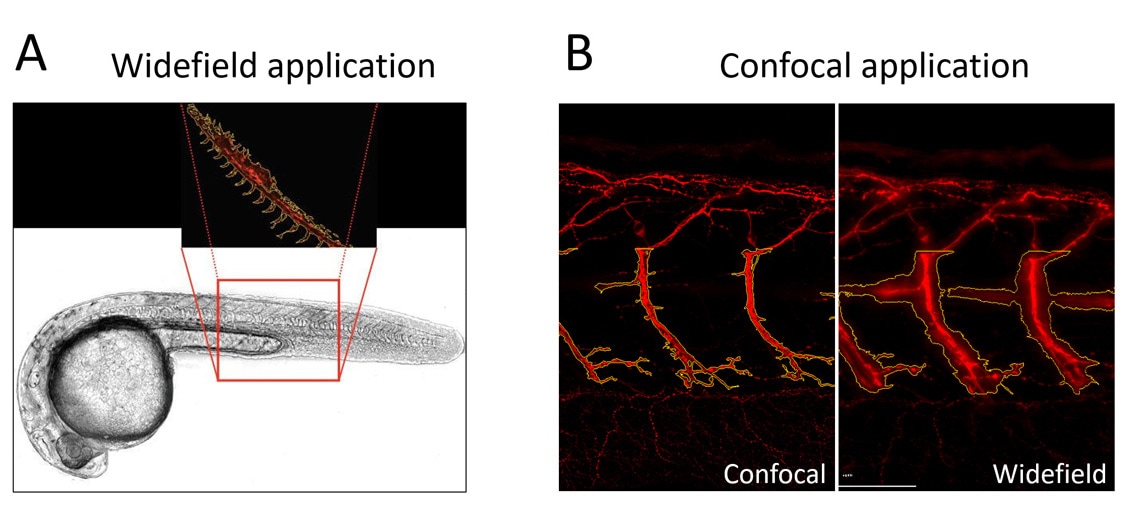
Figure 1. Widefield and confocal fluorescence microscopy applications for zebrafish phenotypic analysis. (A) Widefield fluorescence quantifies total vascular area as shown in this transgenic zebrafish expressing red fluorescence throughout the vasculature. Here widefield is preferred due to the low background fluorescence and large field-of-view (low magnification) desired to capture a large portion of the vascular structure. (B) Confocal fluorescence is preferred to appropriately segment individual neurons (red staining) in this immunofluorescence zebrafish application. For this sample, widefield images include too much out-of-focus background fluorescence to properly segment motor neurons.
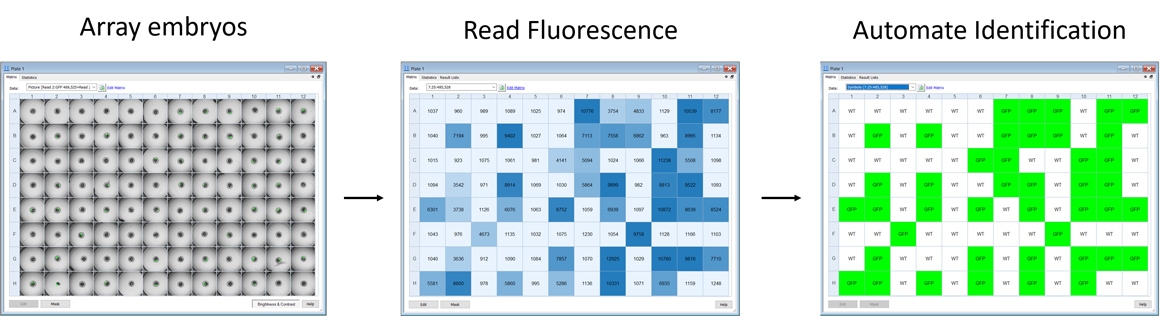
Automate routine fluorescence sorting tasks that don't require imaging
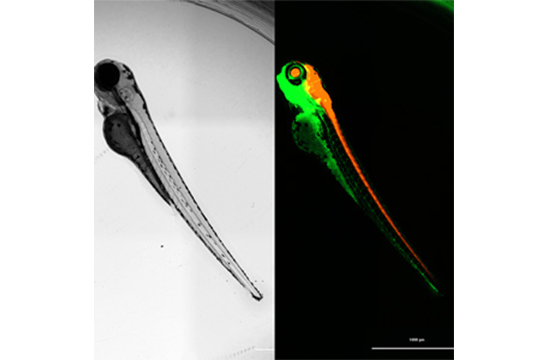
The small size of zebrafish embryos makes them highly amenable to higher throughput formats, such as 96-well microplates. This enables automation of routine tasks like sorting fluorescent embryos from non-fluorescent siblings. Depending on the onset time of fluorescence, embryos can be sorted in their chorions without anesthesia, or immobilized with anesthetics at later stages (post-hatching) for sorting. Round-bottom microplates can be used to effortlessly and automatically position the embryo in the center of each well. Fluorescence detection using plate-reader monochromators can identify fluorescent embryos, such as the transgenic GFP-expressing embryos pictured in Figure 2. Here, 1-day old embryos were arrayed into a round bottom 96-well microplate for sorting by using reader-based fluorescence on the Cytation C10.
Images show arrayed 1-day old zebrafish embryos positioned in the center of the well in a 96-well microplate. Fluorescence detection of all 96 embryos takes approximately one minute, and a cutoff threshold is then applied to identify GFP positive fish from non-expressing siblings.
Customer Spotlight
Zebrafish and Lionheart: A Perfect Match
Dr. Charles Hong, University of Maryland School of Medicine
Zebrafish are essential to Dr. Charles (Chaz) Hong's laboratory at the University of Maryland School of Medicine. Here, these diminutive powerhouses of developmental biology research are used to characterize the chemical biology of vertebrate development, and to also discover novel chemical tools to selectively modulate embryonic development. As a matter of fact, the Hong Lab used zebrafish in their discovery of the world's first bone morphogenetic protein (BMP) inhibitor, called dorsomorphin which was identified by its ability to make the body of the embryos shorter. Dr. Charles (Chuck) Williams, Assistant Professor of Medicine, who works with the Hong Lab, studies embryonic development in zebrafish using a forward chemical genetic approach. This entails identifying small molecule compounds that interact with proteins important in development. He discovers new biological insights based on how the interaction "breaks" the signals that drive zebrafish development.
Developmental Biology Resources
Webinars
Multimodal zebrafish workflows on a single automated confocal microscope plate reader
Presenter: Rebecca Mongeon, PhD., Principal Scientist
Quantitative phenotypic analysis is central to many zebrafish research workflows. Phenotypic analysis often involves leveraging multiple technologies for molecular-, cellular- and organismal-level evaluations.
The newly introduced Cytation C10 confocal imaging reader is a powerful single-instrument solution for multimodal plate reading, widefield fluorescence microscopy, and spinning disk confocal microscopy. Join us in this webinar to explore multimodal zebrafish applications that enable automated zebrafish workflows on a single instrument.
Zebrafish Imaging with Quantitative Analysis On Demand
Presenter: Sarah Beckman, PhD., Principal Scientist
Zebrafish are a powerful vertebrate model for many avenues of biological research, including developmental biology and pathology. Due to their small size and optical clarity, they are uniquely amenable to imaging studies. In this webinar we will describe how automated digital microscopy and quantitative image analysis can be applied to zebrafish research focusing on three common areas of study including angiogenesis, toxicology and cell death.
For Research Use Only. Not for use in diagnostic procedures.