Cell Metabolism
May 2020
Homeostasis of cellular metabolism is vital to maintaining the life of all organisms. A critical part of metabolism is oxidative phosphorylation, a process that uses oxygen to produce energy for the cell in the form of adenosine triphosphate (ATP). Mitochondrial dysfunction or fluctuations in cellular oxygenation as often observed in solid tumors or during ischemia, have been implicated in different disease states. Cancer cells, for example, often show altered metabolism to favor glycolysis over oxidative phosphorylation as a response to low oxygen levels in the microenvironment and to support malignancy. Monitoring cellular metabolism is therefore a central part of understanding disease states and developing effective therapeutics.
Tools that provide ease-of-use, higher throughput, and multiplexed data are important aides in the ongoing study of cellular metabolism. One of these tools is the Agilent Seahorse XF Analyzer, widely accepted as the industry standard for obtaining in vitro cellular metabolic parameters. Cellular metabolic functions are measured as oxygen consumption rate (OCR) and extracellular acidification rate (ECAR), and XF Analyzers report OCR and ECAR for any given sample in microplate format. As for many assays, normalization of the functional data to cell numbers is helpful to ensure accurate and consistent interpretation of the results.
Counting cell numbers per well before or after functional analysis is the most robust normalization method for XF metabolic rate data. Individual cell nuclei in the Seahorse XF assay plate are stained with Hoechst 33342 and capturing microscopic images using a DAPI filter cube on the BioTek Cytation 1 / Cytation 5 Cell Imaging Multi-Mode Readers directly on the assay plate. Gen5 and the Agilent Seahorse XF Imaging and Cell Counting software count the stained nuclei per well and allow to directly normalize Seahorse XF metabolic rate data with just a few simple steps.
In addition to the Seahorse XF instruments, Agilent offers a range of soluble metabolic sensors. The Agilent MitoXpress and pH-Xtra assays are simple plate reader-based mix-and-measure assays compatible with a variety of cellular matrices. These assays utilize soluble fluorescent probes to measure oxygen consumption (MitoXpress Xtra), extracellular acidification (pH-Xtra) or intracellular oxygen concentration (as %O2, MitoXpress Intra) in live-cell monolayers and other biological matrices. BioTek plate readers, such as the Cytation family, the Synergy H1 and Synergy Neo2 Hybrid Multi-Mode Readers with dual read time-resolved fluorescence detection and custom filter sets, are well suited for the measurement of the Agilent soluble metabolic sensors. Tailor-made BioTek Gen5 templates (available for download) for these assays further facilitates streamlined data reduction and analysis, making BioTek plate readers a perfect choice for measuring cellular respiration, glycolytic flux and intracellular oxygenation on a plate reader.
Featured Applications
Optimization of a Multi-Mode Detection Model for Measuring Real-Time Cellular Respiration and Mitochondrial Function using Fluorescent Biosensors
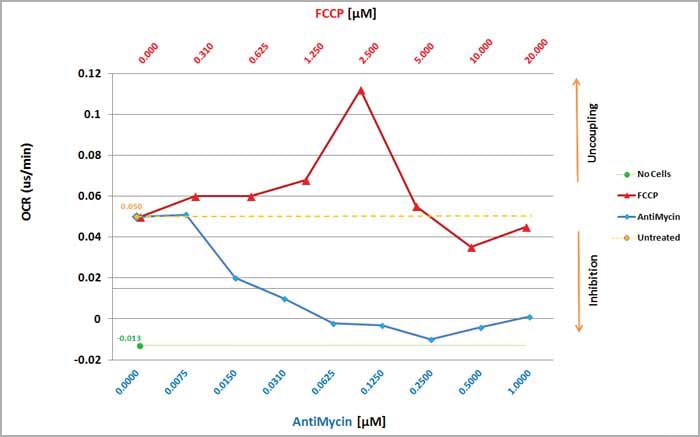
Monitoring cellular metabolism is a central part of understanding disease states and developing effective therapeutics. Changes in mitochondrial function, electron-transport chain activity or glycolytic flux are critical parameters in the functional analysis of cellular metabolism. Using Agilent's family of soluble metabolic sensors (MitoXpress & pHXtra) on BioTek Multi-Mode plate readers facilitates the assessment of these parameters by measuring oxygen consumption, extracellular acidification, or intracellular oxygen levels in live-cell monolayers and other biological matrices.
BioTek's custom filter sets, dedicated Gen5 templates, and time-resolved fluorescence detection technology make it even simpler for researchers to assess cellular metabolism using fluorescent probes with reduced background and increased signal dynamic range.
XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System
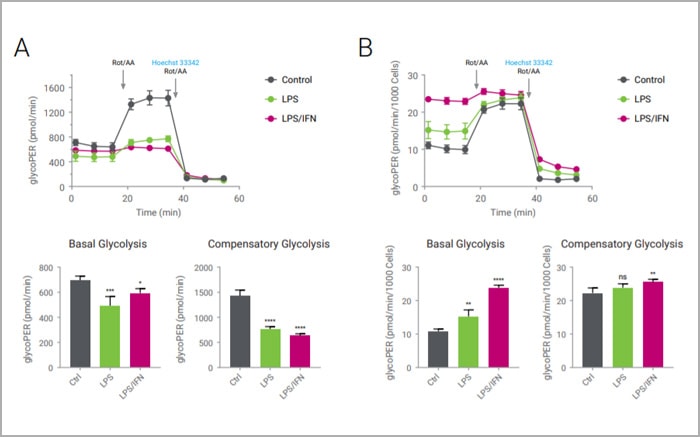
Data normalization is required for any experiment to reach a valid conclusion, particularly when variation in sample quantity is involved. For real-time cellular metabolic analysis using Agilent Seahorse XF technology, the cell quantity per well is a commonly accepted reference value for data normalization. It can be assessed directly by cell counting or indirectly by measuring biomolecules such as total protein or genomic DNA. The Agilent Seahorse XF Imaging and Normalization system integrates XF technology with a Cytation 1/5 Cell Imaging Multi-Mode reader (BioTek Instruments, Inc). In this configuration, the Cytation 1/5 is controlled by the new Agilent Seahorse XF Imaging and Cell Counting software, which provides a simplified, automated, and validated solution for microscopic image-based cell counting. This turnkey solution includes 1) in-situ administration of a cell membrane-permeable nuclear staining compound (i.e. Hoechst 33342), 2) automated imaging and cell counting optimized for XF assays, and 3) synchronization of images and cell counts into XF result files in Wave software. This solution provides an efficient and reliable method of normalization with a seamless and rapid workflow for XF Analyzer users.
Product Spotlight
Cytation 5 Cell Imaging Multi-Mode Reader
Cytation 5 is a modular, upgradable multi-mode reader that combines automated digital microscopy and conventional microplate detection. Cytation 5 includes filter- and monochromator-based detection; the microscopy module provides up to 60x magnification in fluorescence, brightfield, color brightfield and phase contrast. Incubation to 65 °C, shaking and Gen5 software are standard.
Watch this video to learn more about the complete Cytation line of cell imaging multi-mode readers.
Tek Tips
Tip for Agilent soluble metabolic sensors: MitoXpress and pH-Xtra
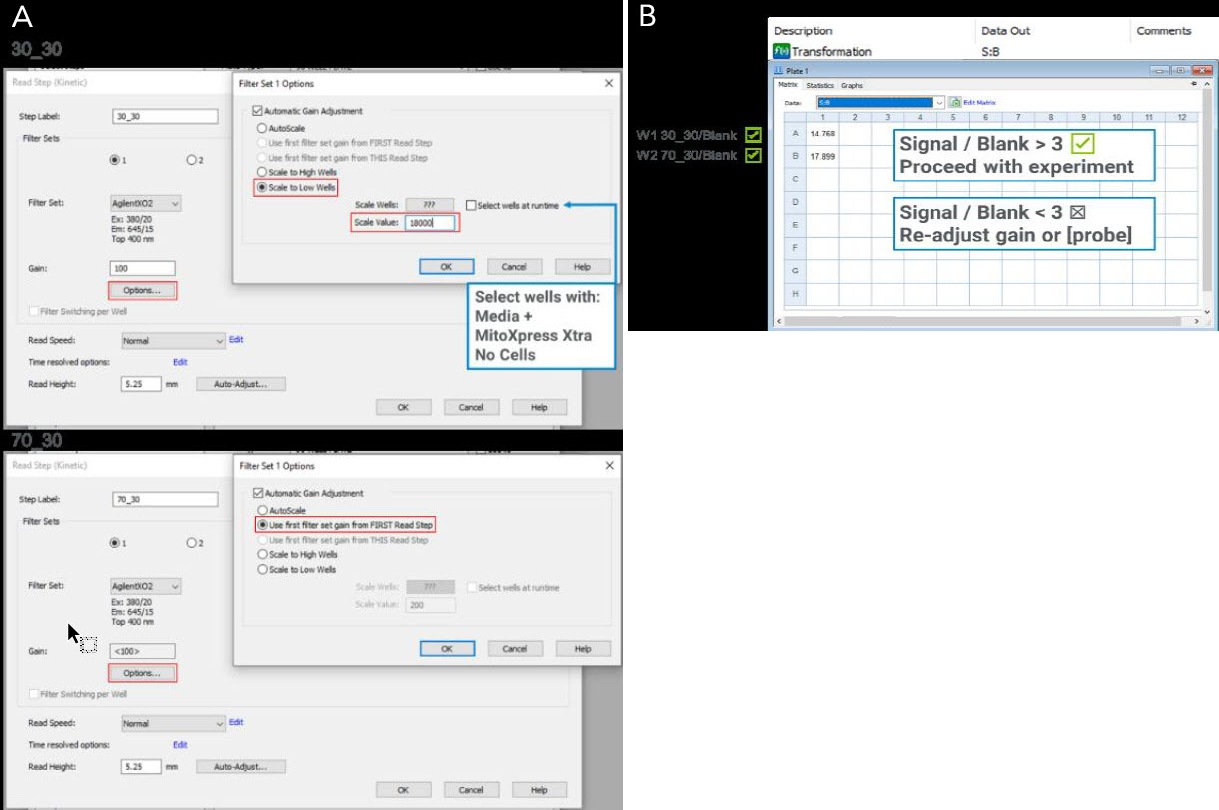
Signal and gain optimization are crucial for optimal assay performance when running an Agilent MitoXpress or pHXtra soluble metabolic sensor assay for the first time. As further described in the kit User Guides, the ratio of the signal from a well containing the metabolic sensor and a blank well (S:B) is used as a metric to estimate whether the optimal settings were chosen.
We recommend that MitoXpress Xtra assays should have at least a S:B of 3. Dual-read TRF measurements, as enabled for many BioTek Multi-Mode readers, are the optimal detection mode for the MitoXpress Xtra Assay and will often deliver S:B ratios well over 3. However, optimization of gain settings is still recommended for optimal assay experience. The simplest way to optimize gain settings for MitoXpress Xtra assays using Gen5 is to follow these steps:
- Open Gain setting options for first TRF step (30_30); select “Automatic Gain Adjustment” and choose wells containing MitoXpress Xtra (but no cells); select “Scale to Low Wells” and set Scale Value to 18000 (or 18% of the maximum intensity for your reader) (Fig.A). Open Gain setting options for second TRF step (70_30); Ensure that the second read (70_30) is set to “Use first filter set gain From FIRST Read Step.”
- Read the wells kinetically for 20 mins to get a stable signal then check the S:B ratio using the pre-defined reduction tab (Fig. B) without causing any signal overflow. If the S:B is <3 proceed to manual adjustment of the gain settings or refer to the kit User Manual.
The gain parameter can be adjusted by repeating the procedure, but for each experiment, only one gain setting should be chosen for both TRF read steps and during the entire kinetic run to ensure correct data reduction.
Automated Imaging and Analysis of Mitochondrial Membrane Potential
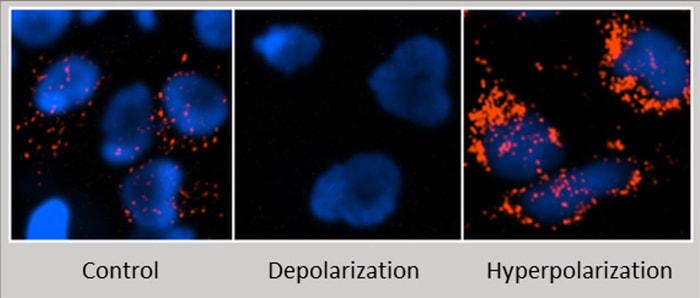
Mitochondria are important cellular organelles responsible for crucial maintenance of energy homeostasis through actions such as ATP generation via oxidative phosphorylation and beta-oxidation of short- and long- chain fatty acids. A variety of measures can be performed to assess the function of mitochondria, including visualizing and quantifying altered mitochondrial membrane potential (MMP). Sustained aberrations in balanced membrane potential (inward transport of cations and outward transport of anions) reflect abnormal physiological conditions. Depolarization may result in targeting of mitochondria for mitophagy and hyperpolarization can coincide with cellular changes that result in necrosis or apoptosis. Therefore, assessment of prolonged changes in polarization may provide insight into these occurrences in your cells. A common method for measurement of mitochondrial membrane potential includes addition of cationic fluorescent probes to cells and image capture.
Both low and high potential mitochondria will accumulate MITO-ID fluorescent dye however the photophysical properties of this reagent cause an emission shift and red fluorescence is only readily detectable in hyperpolarized conditions. Images of Hela cells were captured using a BioTek automated imaging instrument after the addition of MITO-ID and a nuclear stain (blue). Visible in this panel are control, depolarized and hyperpolarized mitochondria of Hela cells.
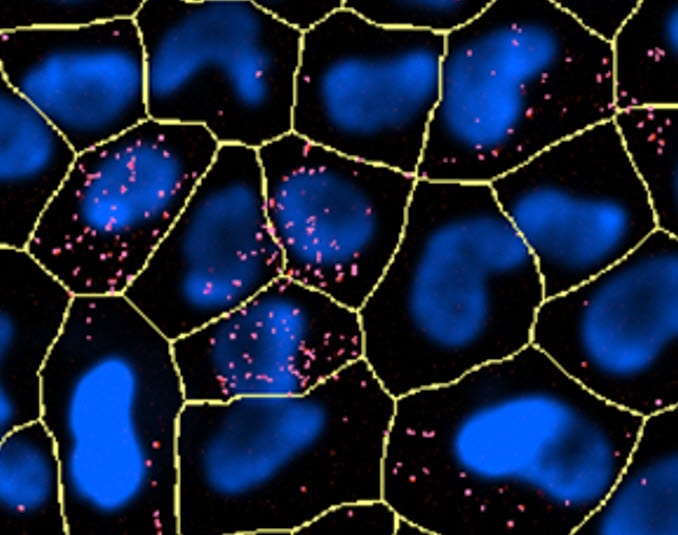
Figure 2. Display of Gen5 analysis masking.
After imaging in two channels and background subtraction, primary masks should be applied to identify all cells in the field of view utilizing the nuclear stain (blue). Secondary masking can then be applied using the red channel.
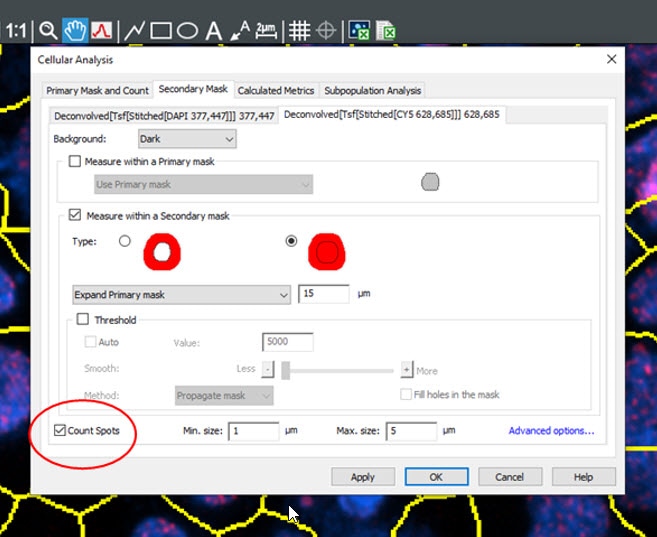
Figure 3. Gen5 image analysis window.
Within the Cellular Analysis window, the spot counting module can be utilized to identify punctae indicative of depolarized mitochondria. This will allow for identification of metrics such as “spots” or MMP positive aggregates per cell.
Here, the use of Gen5 Image Prime and the additional Spot Counting module allow for accurate and efficient image analysis to provide identification of aberrant mitochondrial conditions via object level metrics including MMP positive aggregate count per cell. Additionally, mean red fluorescence, and integral/sum integral red fluorescence can be utilized as metrics on a per cell basis to provide supplementary data for comparisons of experimental and control conditions. Important considerations for all experimental measures related to cellular metabolism include timings of treatment and probe addition, cellular confluency at the time of imaging and measurement as well as culture media conditions. It is important to consider how each of these factors may affect your experiment and measurement outcomes as adjustment of these factors may be required to achieve the best experimental measurements.
Cell Metabolism Resources
Application Notes
- Optimization of a Multi-Mode Detection Model for Measuring Real-Time Cellular Respiration and Mitochondrial Function using Fluorescent Biosensors
- Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation 5
- XF Data Normalization by the Agilent Seahorse XF Imaging and Normalization System
Technical Note
Application Bulletins
Webinars
Moving Cell Migration Research Forward with Integrated Solutions by BioTek Instruments
Presenters: Dr. Samila Nasrollahi and Dr. Tom Lampert
This webinar will explore common cell migration assays including wound healing, transwell migration, and three-dimensional invasion. Specifically, this will provide automated solutions for these procedures to increase workflow and improve quantitative parameters.
Using Automated Imaging to Quantitatively Analyze the Colony Formation Assay
Presenter: Dr. Ernest Heimsath; Principal Scientist
In this webinar, we discuss automated methods for conducting and analyzing the colony formation assay using both the colorimetric and fluorescent properties of crystal violet, the well-established dye for this assay. We will cover important considerations for setting up the colony formation assay in multi-well formats, as well as discuss approaches for constructing a quantitative image analysis workflow that reliably identify colonies and extract meaningful results.
Ask the Experts – Studying Cellular Metabolism in Cancer and Beyond - On Demand
Presenters: Dr. Valerie Sodi and Dr. Amanda Herberger, Field Applications Scientists, BioTek Instruments, Inc.
Ask the Expert Series: BioTek's U.S. FASTeam talks about best practices, troubleshooting, assay design, and experimental optimization. These discussions are heavily based on audience questions. Series moderated by Dr. Diane Kambach, Team Leader of the BioTek FASTeam.
Containment Enclosures and Biosafety Cabinets for
BioTek Instrumentation

The emergence of automated equipment for cell culture research has required creative solutions to provide isolation of cell lines and containment of potentially hazardous agents. Automated equipment size requires larger workspaces that can accommodate fluidics, power requirements, heat rejection, service and user interface ergonomics.
BioTek Instruments and The Baker Company have established a field collaboration providing the automated lab with the best solution possible in innovative life science instrumentation and containment control for a wide variety of applications.
Contact your BioTek Sales Representative with any questions.
For Research Use Only. Not for use in diagnostic procedures.