Cell Counting
May 2021
The ability to accurately determine cell number is an important aspect of a broad range of applications, including setting up and optimizing cell-based assays, normalizing data across samples, and conducting cell proliferation assays. The BioTek Lionheart FX Automated Microscope and Cytation Cell Imaging Multi-Mode Readers utilize label-free and fluorescence imaging modes, along with powerful Gen5 image analysis tools, to deliver efficient and effective automated cell counting.
Here we've compiled resources that provide guidance on conducting and optimizing image-based cell counting applications, as well as important considerations for selecting the most effective cell counting technique.
Featured Applications
Automated Kinetic Imaging Assay of Cell Proliferation in 384-Well Format
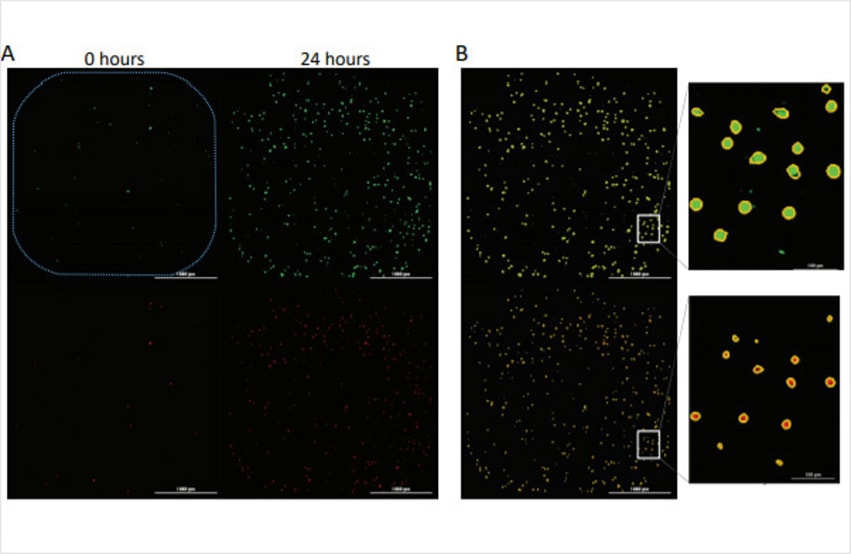
Evasion of cell death is a hallmark of cancer cells that allows them to overcome endogenous barriers to cancer development, as well as to resist treatment. Understanding how cancer cells are able to evade cell death promises to unlock new avenues to treat human cancer. Here we present a fully automated image-based assay to monitor both the proliferation and cell death of a fibrosarcoma cancer cell line in response to multiple anti-neoplastic drugs in a high throughput format.
Confocal Imaging and Analysis of Spheroids for Determination of Dose Response during Drug Treatment
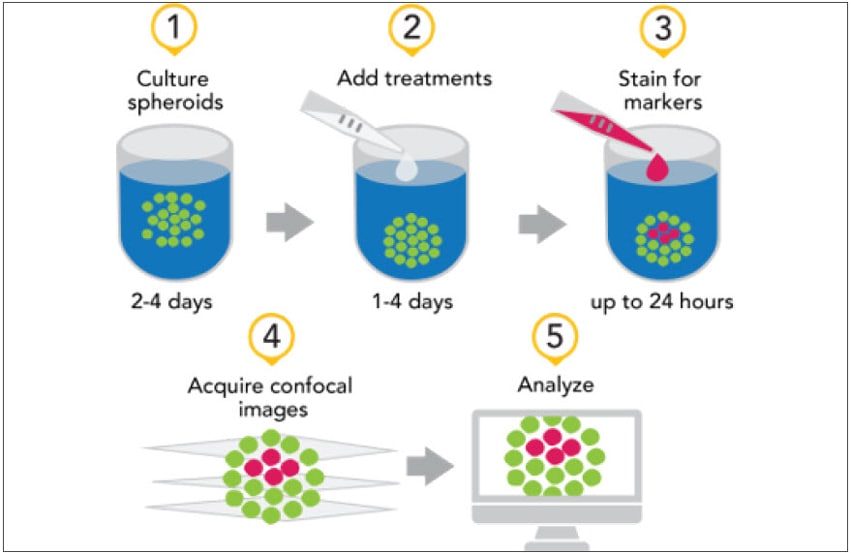
The last decade has seen an explosion in the use of cells and tissues from a wide range of sources cultured in a three-dimensional (3D) setting resulting in more complex biological models. The application of these 3D models has been on the increase in areas such as medical research, precision medicine, disease modeling and drug discovery efforts. These models tend to be representative of the native microenvironments found in organisms and are thought to provide a more accurate assay model in some instances. 3D models are often compared to the widely used two-dimensional (2D) models, those consisting of a monolayer of cells, which have been in use for decades.
Product Spotlight
Cytation C10 Confocal Imaging Reader
Cytation C10 Confocal Imaging Reader combines automated confocal and widefield microscopy with conventional multi-mode microplate reading. The spinning disk confocal module adds increased resolution and optical sectioning capabilities to the Cytation range. Cytation C10 also includes widefield fluorescence, brightfield and phase contrast optics. The multi-mode module has variable bandwidth monochromator-based optics for specificity and sensitivity. System control, image and data analysis provided by BioTek's Gen5 software.
Cytation C10 Confocal Imaging Reader
Cytation Cell Imaging Multi-Mode Plate Readers: Ready for Any Assay
Tek Tips
Evaluating label-free and nuclear label-based cell counting techniques
Determining cell counts throughout live tissue culture experiments is an essential aspect to most imaging assays. Vital fluorescent stains are often employed for this purpose, but they can introduce a risk of toxicity and confounding effects to the biology under investigation. Consequently, non-perturbing methods are highly valuable, such as BioTek's label-free high-contrast brightfield method (HCBF). The principle of high-contrast cell counting relies on using defocused illumination to make cells appear as singular bright spots that are easily counted. The efficacy of HCBF, including the intensity and shape of the bright spots it produces, is dependent on the cellular morphology. Cells that are more spherical in morphology will appear as bright spots closer to the focal plane than cells that are flatter in morphology. In practice, there is a range of HCBF offsets where many cell types can be successfully quantified using HCBF. However, the sensitivity of HCBF to cell morphology can also lead to situations where HCBF is not ideal for evaluating cell counts.
First, some cell types will not be amenable for HCBF counting when they display interfering morphologies and tendencies in culture (ie. clustering and pileup) (Figure 1). Second, some treatments alter cell morphology in a way that dramatically changes the flatness of the cell. For example, treatments resulting in apoptosis can induce cell blebbing and fragmentation of cells into multiple spherical cytoplasm blebs. In this case, HCBF may quantify multiple cell fragments, leading to apparent increases in cell counts until these fragments necrose or fully disperse into debris. Although it may be possible to optimize HCBF to reduce the effects of fragmentation or morphology changes (generally by bringing the HCBF focal plane offset closer to the sample plane), vital dyes remain a consideration for achieving accurate counts for some assays.
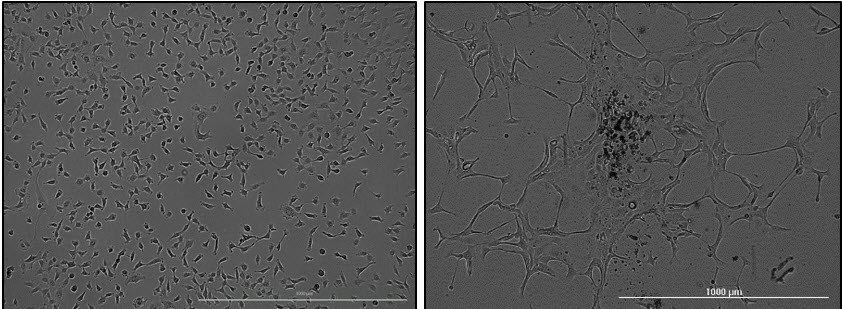
Figure 1. Recognizing some cell types are less amenable to label-free cell counting techniques. Individual HT-1080 cells (left) are readily identifiable due to their rounded shape and tendency to remain evenly distributed. In contrast, Hs 578T cells (right) tend to cluster and present a flatter morphology that is less amendable to resolving individual cells using label-free cell counting techniques.
Optimizing staining protocols to promote reliable cell counts while minimizing cell perturbation
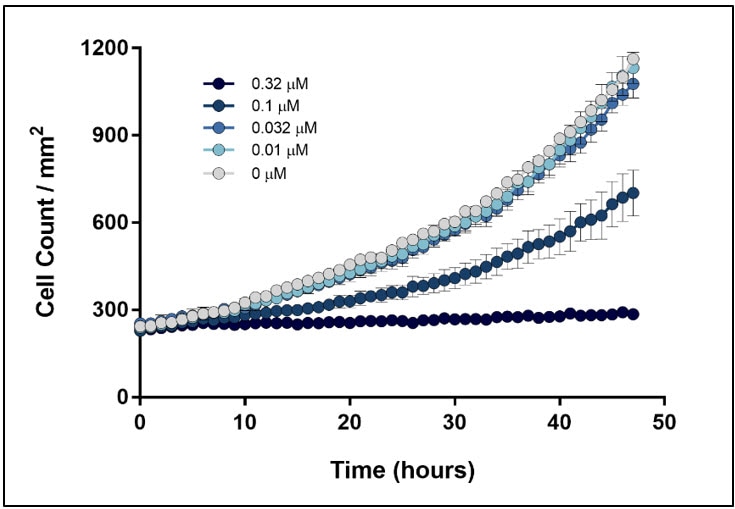
If dyes are required for cell counting, minimizing the risk of confounding effects to the biology being studied is a top priority. A broad range of fluorescent stains are available for directly counting cells that are not amenable to label-free counting methods. One of the vital dyes often employed for cell counting is the DNA-binding dye Hoechst, as tissue culture cells are often uninucleate and nuclei counts correlate well to cellular counts. However, the addition of these reagents can produce unintended cell type-dependent cytotoxic effects. Gen5 image analysis tools provide the ability to determine the appropriate concentrations of reagents to achieve efficient labeling through the experiment without significantly altering cellular behavior in the process. In the following example, HT-1080 cells were stained with a range of Hoechst 33342 concentrations to determine an effective staining protocol to accurately measure treatment-induced changes in HT-1080 proliferation rates. The minimal amount of stain necessary to effectively identify and count each cell was determined by imaging HT-1080 stained with a range of Hoechst 33342 concentrations over a 48-hour time course and then calculating the signal to noise ratios over time (Figure 2).
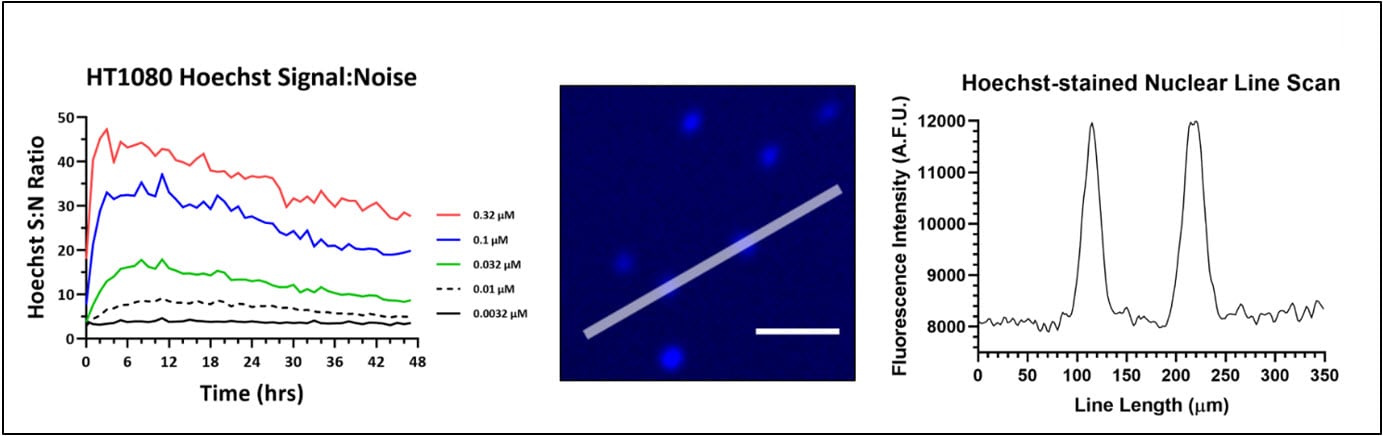
Figure 2. Hoechst 33342 optimization in a 48hr proliferation assay using HT-1080 fibrosarcoma cells. (A) Signal-to-Noise (S:N) ratio of Hoechst 33342 in proliferating HT-1080 cells over time. Signal was derived by setting a primary mask around each nucleus, while background was measured using a secondary mask that extended 5 µm beyond the primary nuclear mask. The S:N value is the mean nuclear signal divided by the square of the standard deviation of the background signal. The S:N ratio at each concentration in the plot represents the mean S:N ratio for all nuclei counted within a 4x image. (B) HT-1080 nuclei imaged using a Cytation 5 after 12 hr incubation with 10 nM Hoechst 33342. The line represents a line scan to assess fluorescence intensity of signal above background. (C) A 350 um line was drawn across two HT-1080 nuclei and their fluorescence intensity profile was plotted to demonstrate sufficient signal of Hoechst 33342 at 10 nM.
The proliferation profiles generated for each population of stained cells were compared to test for any unintentional effect on HT-1080 viability and growth kinetics (Figure 3). From these results, it was determined that 32 nM Hoechst 33342 delivered efficient staining throughout the specified time course without altering cell viability and proliferation rates.
Figure 3. Effect of Hoechst 33342 concentrations on HT-1080 proliferation kinetics. HT-1080 stained with a range of Hoechst 33342 were monitored over 48 hours to test for unintended effects on cell viability. Label-free high contrast cell counting was used to measure negative control conditions (0 µM).
Cell Counting Resources
Application Notes
- Kinetic Proliferation Assay Using Label-Free Cell Counting
- Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation 5
Application Bulletin
Technical Notes
- Automated Cell Counting with High Contrast Brightfield and EVE Counting Slides
- High Contrast Brightfield
Webinars
Tools and Techniques for Optimizing Cell Proliferation Studies - On Demand
Presenter: Joe Clayton, Senior Principal Scientist
QuantitativeIn this webinar we provide a detailed review of available methods for measuring cell proliferation, with a particular focus on recent assay innovations, including both 2D and 3D formats. This information will assist researchers in selecting the most appropriate and effective system for measuring cell proliferation based on a broad range of factors. Additionally, we cover important considerations for setting up and running cell proliferation assays, including techniques for improving the accuracy and reproducibility of results.
For Research Use Only. Not for use in diagnostic procedures.