Advancing Cancer Cell Invasion and Metastasis Research with Powerful Cell Analysis Tools
January 2025
Cell migration and invasion are critical processes in cancer metastasis, where cancer cells spread from the primary tumor to distant sites in the body. Understanding these mechanisms is essential for developing effective cancer therapies. Traditional methods for studying cancer cell invasion often face limitations, relying on endpoint measurements and low-throughput approaches which can impede research progress.
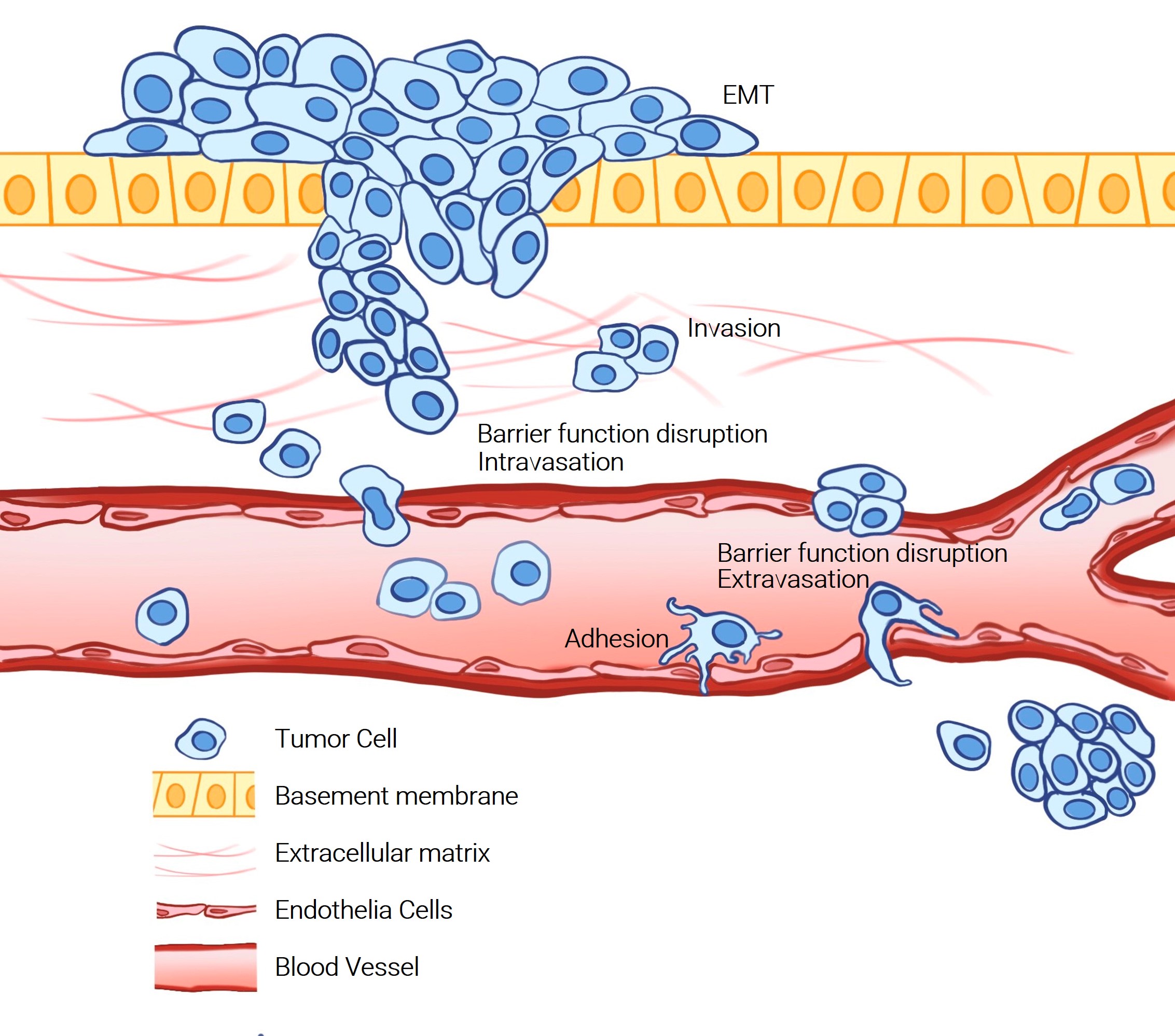
Agilent addresses these challenges by offering automated, high-throughput, and real-time monitoring capabilities, delivering researchers with precise and reproducible data. Their innovative approaches, including automated imaging systems and impedance-based formats, enable detailed analysis of cancer cell behavior in both 2D and 3D environments. These solutions support a wide range of applications, from EMT transition, cell invasion and migration, barrier function disruption and cell adhesion, enhancing the accuracy and efficiency of experiments.
In this TekTalk, we’ve assembled diverse resources for conducting and optimizing assays for measuring cell invasion behavior utilizing Agilent’s unique portfolio of cell analysis technologies.
Featured Application Notes

High-Throughput Methods to Quantitatively Evaluate Cell Signaling in Epithelial-to-Mesenchymal Transition
This application note discusses high-throughput methods to quantitatively evaluate TGF-β signaling in epithelial-to-mesenchymal transition (EMT), a process where epithelial cells acquire mesenchymal properties, aiding in cancer metastasis. The study utilizes the Agilent BioTek Cytation C10 confocal imaging reader and Gen5 microplate reader to analyze TGF-β/SMAD pathway activation in A549 lung carcinoma cells. The methods include biochemical, cellular, and multicellular (2D and 3D spheroid) models to assess SMAD phosphorylation and EMT-associated gene expression. The findings demonstrate dose-dependent TGF-β1-induced SMAD2/3 phosphorylation and nuclear translocation, as well as increased expression of EMT markers, providing a comprehensive approach for studying EMT in cancer research.
Dynamic Monitoring of Cell Adhesion and Spreading
This application note highlights the efficiency of the impedance-based xCELLigence RTCA DP system with CIM-Plate for real-time monitoring and quantitative assessment of cell attachment and spreading. Unlike conventional methods, this system eliminates the need for labor-intensive and costly cell labeling, making it faster and more economical. Its noninvasive nature allows users to observe the effects of matrix proteins on adhesion, spreading, and other biological events, such as differentiation and proliferation, within a single experiment. Traditional methods would require separate experiments for each event, making this system a more streamlined and versatile solution.
Tek Tips
General considerations for optimizing cell migration and invasion assays incorporating ECM
The extracellular matrix (ECM) is a complex network of proteins and polysaccharides that provides structural and biochemical support to surrounding cells. It includes components like collagen, elastin, fibronectin, and laminin, which form a scaffold that influences cell behavior, including adhesion, migration, and differentiation[1]. In cancer cell invasion and metastasis assays, the ECM mimics the in vivo environment, making it crucial for accurate and reproducible results.
For optimizing these assays, consider the following technical guidance:
Matrix composition:
Choose the appropriate ECM components based on the specific cancer cell type and the biological question. For instance, Matrigel, a basement membrane matrix, is commonly used for invasion assays due to its rich composition of ECM proteins [2].Coating consistency:
Ensure uniform coating of the ECM on assay plates. This can be achieved using Agilent BioTek automated liquid handling systems, which provide precise and reproducible dispensing of ECM solutions [2].Cell seeding density:
Optimize cell seeding density to prevent overcrowding, which can lead to nutrient depletion and altered cell behavior. Characterization of single-cell migration and invasion requires a low cell density to track and measure individual cells, whereas collective cell migration studies typically rely on a uniform confluent layer of cells [2].
By carefully considering these factors, you can enhance the reliability and accuracy of your cancer cell invasion and metastasis assays.
Product Spotlights
Agilent BioTek Cytation C10 Confocal Imaging Reader
The BioTek Cytation C10 confocal imaging reader integrates confocal and widefield imaging with multimode microplate reading. The imaging capability enables a range of methods in cancer metastasis research, including 3D cell migration and invasion assays, along with 2D scratch wound healing, barrier exclusion assays, and transwell assays.

xCELLigence Real-Time Cell Analysis (RTCA) DP
The RTCA DP system in combination with CIM-Plate 16 devices allows label-free, automated quantification of cell migration and invasion in real time under physiological conditions. Each well in the CIM-Plate 16 is a modified Boyden chamber. The impedance biosensor on the porous membrane automatically detects cells as they migrate through the porous membrane and attach to the impedance microelectrode in the lower chamber. The RTCA software creates the kinetic migration curves automatically.

Webinars OnDemand
Identifying Regulatory Mechanisms in Cancer Metastatic Processes with an Integrated Multi-omics Approach
In this webinar, Dr. Steven Offer from the University of Iowa, formerly of the Mayo Clinic, discusses a comprehensive multi-omics approach to identifying regulatory mechanisms in cancer metastasis. By applying this method to study colorectal cancer invasiveness in a controlled experimental setting, key regulators of this process were identified. The xCELLigence Real-Time Cell Analysis technology was deployed to measure cell proliferation, migration, and invasion, helping to identify regulators of cell invasiveness in a colorectal cancer model.
Navigating the cell migration assay landscape: How to bring together the most appropriate tools and approaches to ensure robust, accurate results
In this Agilent Cell Analysis webinar, we will provide a review of existing assay formats for studying cell migration behavior, including the scratch wound healing assay, barrier cell exclusion formats (e.g., Oris and Ibidi), chemotaxis assays, and 3D cell culture formats for characterizing cell invasion. We’ll provide guidance on selecting the most appropriate assay format considering several factors, such as biological context/sample type, throughput, cost, and desired readout.
Additional Resources
- xCELLigence RTCA Wet Lab Tutorial: Performing Cell Invasion/Migration Assay
- xCELLigence – eSight – scratch wound: Live Cell Analysis of Scratch Wound Migration and Invasion Healing Assays using the Agilent xCELLigence RTCA eSight
- Real-Time Monitoring and Quantifying 3D Tumor Spheroid Invasion Using the Agilent xCELLigence RTCA eSight System
- Combination of a Fluorescent Substrate-Based MMP Activity Assay and Hit Pick Reading/Imaging Procedure
- Automated, Kinetic Imaging of Cell Migration and Invasion Assays Using Corning FluoroBlok Permeable Supports
- Automated Monitoring of Protein Expression and Metastatic Cell Migration using 3D Bioprinted Colorectal Cancer Cells
- Hou, Xiangchan et al. “KCNK1 promotes proliferation and metastasis of breast cancer cells by activating lactate dehydrogenase A (LDHA) and up-regulating H3K18 lactylation.” PLoS biology vol. 22,6 e3002666. 21 Jun. 2024,
- He, Y., Shi, Q., Ling, Y. et al. ABLIM1, a novel ubiquitin E3 ligase, promotes growth and metastasis of colorectal cancer through targeting IĸBα ubiquitination and activating NF-ĸB signaling. Cell Death Differ 31, 203–216 (2024).
- Godet, I., Shin, Y.J., Ju, J.A. et al. Fate-mapping post-hypoxic tumor cells reveals a ROS-resistant phenotype that promotes metastasis. Nat Commun 10, 4862 (2019).
- Gallanis, G.T., Sharif, G. et al. Stromal Senescence following Treatment with the CDK4/6 Inhibitor Palbociclib Alters the Lung Metastatic Niche and Increases Metastasis of Drug-Resistant Mammary Cancer Cells - PubMed Cancers 15(6):1908 (2023)
Upcoming Events
- SLAS (January 25-29, 2025)
- SITC Cellular Therapy for Solid Tumors in San Diego (March 11-14, 2025)
- SOT (March 16-20, 2025)
- AACR (April 25 - 30, 2025)
For Research Use Only. Not for use in diagnostic procedures.
RA45659.3996412037