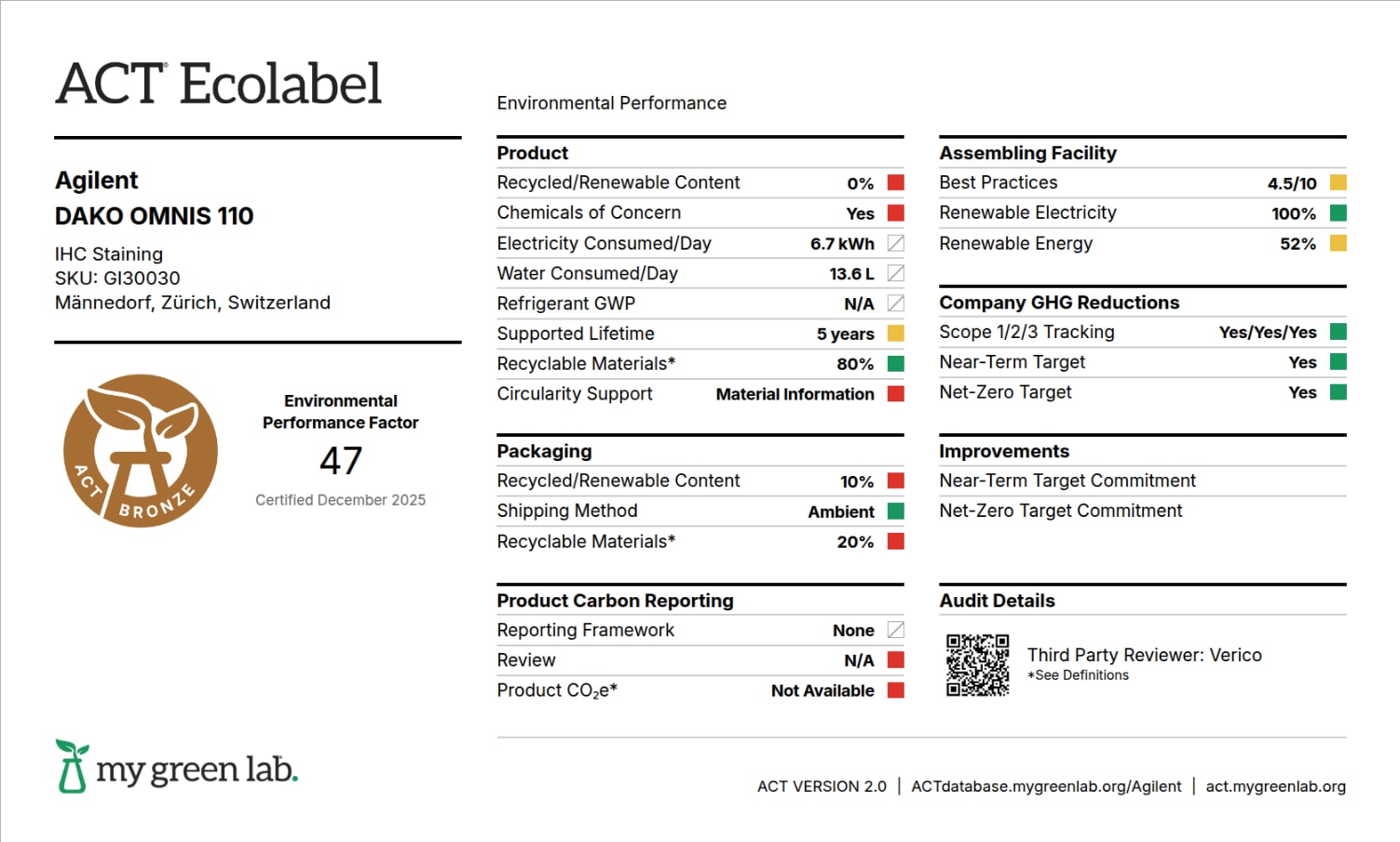
Dako Omnis 110
Redefine your advanced tissue staining productivity with continuous processing of patient cases, which will help you achieve more. Get increased throughput with fewer instruments and less hands-on time, while at the same time improving the case turnaround time. By arranging the optimized workflow around the diagnostic unit—the patient case—Dako Omnis enables faster and continuous delivery of complete cases.
The Dako Omnis 110 provides sustainable design features such as:
- Five years supported lifetime
- 80% recyclable instrument materials driven by steel (70%) and aluminum (10%)
- Manufacturing facility uses 100% renewable electricity

Supporting Information/Audit Definitions
Product
Recycled/Renewable Content
- The total Product Recycled/Renewable Material Content is rounded down to the nearest 10%.
- The product contains sourced components with recycled/renewable content.
Chemicals of Concern
- The product has at least one chemical on the EU REACH SVHC Candidate List, or information was not provided.
The product is compliant with the EU RoHS Directive (or equivalent).
Electricity Consumed/Day
- Electricity consumption is determined by the product performance in a typical use case scenario. The use case scenario represents one of many possible uses and work flows for any product that is tested.
- The product consumes 6.7 kWh in a typical day, as defined in use case scenario provided in the My Green Lab testing protocol or approved industry standard.
- Equipment does not include an automatic power-saving mode that can be activated by the user.
- Equipment does not include an automatic shutdown mode that can be activated by the user
- Equipment does not include an automatic shutdown mode as a manufacturer default.
- Protocol used by the manufacturer for energy metering of the equipment: My Green Lab-approved Test Protocol
Water Consumed/Day
- Water consumption is determined by the product performance in a typical use case scenario. The use case scenario represents one of many possible uses and work flows for any product that is tested.
- The product consumes 13.6 L of water in a typical day, as defined in the use case scenario provided in the My Green Lab testing protocol or approved industry standard.
Refrigerant GWP
- The product does not use a chemical refrigerant or chemical insulator.
Supported Lifetime
- The most common length of product support by the manufacturer is 5 years.
- The company provided information on product lifetime support that is available in key markets.
Product End of Life Recyclable and Compostable Materials
- Note that the presence of commonly recyclable or compostable material(s) in the product does not confirm that the material(s) may be readily recycled or composted in the form/configuration found in the product. The total Product End of Life Recyclable and Compostable Materials are rounded down to the nearest 10%.
- The product contains commonly recyclable materials including Aluminum, Steel (all types), and HDPE (#2).
Circularity Support
- The company provided information on circularity support that is available in key markets.
Packaging
Recycled / Renewable Content
- The total packaging recycled/renewable material content is rounded down to the nearest 10%.
- The packaging contains sourced components with recycled and/or renewable content.
Shipping Method
- The product is shipped at ambient temperature.
Packaging End of Life – Recyclable and Compostable Materials
- The presence of commonly recyclable or compostable materials does not confirm they can be readily recycled or composted in their current form or configuration.
- The packaging contains commonly recyclable materials, including: PET / PETE (#1), HDPE (#2), Cardboard
Product Carbon Reporting
- Available cradle-to-gate product CO₂e results may be used for estimates supporting Scope 3 calculations. Note that Product carbon footprint results may not be directly comparable between products in the absence of a Product Category Rule (PCR), even when using an approved reporting framework.
Facility
Best Practices – Culture of Sustainability
- The facility has guidelines for Design for the Environment (DfE), sustainability goals, or sustainability templates used during the product development process.
- The facility provided square footage and product information to improve understanding of energy intensity in life sciences manufacturing.
Best Practices – Climate and Energy
- The facility has reduced greenhouse gas (GHG) emissions by at least 8.8% annually from a qualifying baseline year.
Best Practices – Waste Diversion
- The facility has reduced waste sent to landfill by implementing waste reduction and diversion initiatives.
- Facility waste and diversion data are tracked at least annually.
Best Practices – Water Use Reduction
- Water use reduction initiatives are implemented at the facility.
Renewable Electricity
- The facility sources renewable electricity using financial mechanisms, including: On-site Power Purchasing Agreements (PPA), Utility Contracts
Renewable Energy
- Total facility energy use includes electricity consumption and non-electricity energy sources.
- Non-electricity energy sources may include fuels used for heating or renewable energy sources.
On-site Renewable Electricity / Energy Generation
- The facility generates renewable electricity on-site.
Manufacturing / Assembly Facility
- The facility of origin is best described as an assembling facility.
- Less than 60% by weight of the product is manufactured on-site.
- Certain product components are sourced from off-site locations.
Company GHG Reductions
Scope 1 / 2 / 3 Tracking
- The company tracks Scope 1 and Scope 2 GHG emissions.
- Scope 1 and Scope 2 GHG emissions are third-party verified to limited assurance or better.
- The company tracks Scope 3 GHG emissions.
- All relevant Scope 3 categories have been identified.
- Scope 3 GHG emissions are third-party verified to limited assurance or better.
GHG Reduction Targets
- The company has publicly disclosed GHG reduction targets.
- Targets are aligned with Near-Term (2030) and/or Net-Zero (2050) guidance.
- The company has a Near-Term GHG reduction target.
- The Near-Term target has been validated by a qualified standard-setting organization.
- The company has a Net-Zero GHG reduction target.
- The Net-Zero target has been validated by a qualified standard-setting organization.
Improvements
Recent Product Improvements
- Recent product-level sustainability improvements have been implemented.
Recent Facility Improvements
- Recent facility-level sustainability improvements have been implemented.
Recent Company Improvements
- The company has added a publicly announced Near-Term target commitment aligned with SBTi or another credible target-setting organization within three years prior to the year of audit.
- The company has added a publicly announced Net-Zero target commitment aligned with SBTi or another credible target-setting organization within three years prior to the year of audit.
For In Vitro Diagnostic Use.
DO141554_1.00