Access Agilent eNewsletter November 2016
Agilent collaborates with BioPharma thought leaders in India to advance biosimilar characterization
Ravindra Gudihal
Agilent Market Development Application Scientist
Debdip Ghosh
Agilent Bio-Columns Product Specalist
Globally, BioPharma companies have begun to improve their analytical divisions in order to remain competitive in the market, as well as meet stringent regulatory requirements. In India, the market is focused on the manufacture of biosimilars—replicas of the original innovator protein therapeutic molecules. Due to different processes employed, biosimilars are not an exact copy of the innovator protein, but have very similar efficacy to the originator molecule. Several analytical techniques, such as LC, LC/MS and spectroscopy, have become important and valuable tools in demonstrating similarities between the innovator and its biosimilar product.
Exploring BioPharma innovation and expansion in India
Seeking to address the unique needs of the Indian BioPharma industry, Agilent hosted a BioPharma Workflow Solution workshop at its state-of-the-art Bangalore site to explore biosimilar characterization. The goal of the workshop was to demonstrate Agilent’s BioPharma Workflow solutions to customers involved in characterization of biopharmaceuticals from discovery to development and in quality control. The event was well attended by thought leaders from the emerging BioPharma industy in India, and provided a robust platform for collaboration with industry experts.
Two-day workshop provides opportunities for learning, idea sharing, and collaboration

Figure 1. Biopharma workshop sessions were held at the Agilent Center of Excellence lab (left) and at the Market development Centre India (right).
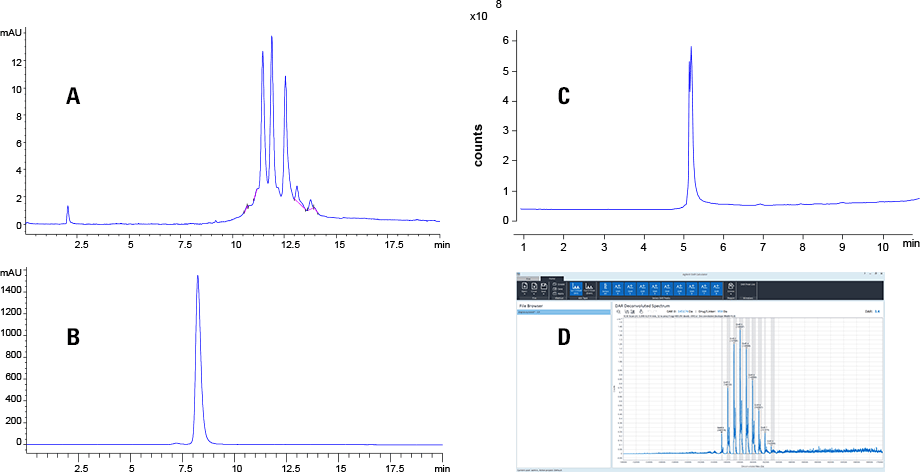
Figure 2. Results from the demonstrations of some workflows during workshop: (A) Charge variant analysis; (B) Size exclusion analysis of ADC; (C) Reversed-phase LCMS analysis of mAb; and (D) Drug antibody ratio analysis using Agilent’s DAR calculator.
More than 25 delegates attended the two-day workshop, which included experts in R&D, method development scientists, downstream scientists, and QA/QC analysts from participating companies. Following an introductory session, topics related to characterization of biomolecules were presented. An engaging Q&A session provided an opportunity for all attendees to share ideas and learnings. As monoclonal antibodies(mAbs) command a major share in market of the biotherapeutics, the primary focus of the sessions was the characterization of protein molecules. Attendees participated in multiple activities conducted in the laboratory (Figure 1). Highlights included examples and application of workflows for peptide mapping, aggregate analysis, change variant analysis, and intact analysis.
Peptide mapping workflow demonstrated on Agilent 1290 Infinity II LC system
Peptide mapping of proteins is performed to confirm sequence identity and is one of the most useful methods to show comparability between innovator and biosimilar. During the workshop, peptide mapping was demonstrated using data generated on Agilent 1290 Infinity II LC system with AdvanceBio peptide mapping column coupled to Agilent 6550 Accurate-Mass Q-TOF LC/MS with iFunnel technology. Data analysis using MassHunter BioConfirm software was demonstrated with its unique features, such as sequence matching and disulfide mapping (Figure 2).
Size exclusion chromatography delivers superior aggregate analysis
Aggregate analysis is an important critical quality attribute (CQA) of protein biotherapeutics. The gold standard for analysis of aggregates in the sample is size exclusion chromatography (SEC). Participants in the workshop experienced a live demo of mAb sample analysis using the newly introduced Agilent AdvanceBio SEC column with Agilent 1260 Infinity II BioInert LC system.
Charge variant analysis workflow demonstration provides insight
Charge variant analysis is another CQA for mAbs. The workflow involves using an ion exchange (IEX) column to separate charge variants using either salt gradient or pH gradient to elute out the different variants from Agilent Bio-IEX columns. Charge variants can occur in molecule due to modifications during storage or handling, such as oxidation and deamidation.
In this workshop, charge variants from one of the biosimilar mAbs were demonstrated using the Agilent 1290 Infinity II 2D-LC Solution. Ion exchange column was in 1st dimension followed by reverse phase in 2nd dimension. Data was presented on 2D-LC/MS for the same molecule. An ideal tool for generating pH or ionic strength gradients for protein charge variant separation is Agilent Buffer Advisor Software.
LC/MS—Best choice for intact and fragment analysis
Intact analysis involves the molecular weight confirmation of the protein molecule (mAbs) with its different post translational modification. The ideal choice of such analysis is LC/MS. A live demonstration of Agilent AdvanceBio Reverse Phase/PLRP-S Columns coupled to Q-TOF showcased better peak shapes and resolution using mAb as an example. Mass analysis was done through MassHunter BioConfirm software. Additionally, glycan analysis using AdvanceBio N-Glycan Sample Preparation Kit was presented to demonstrate the analysis of glycan from mAbs.
Leading professor delivers insightful talk on challenges faced by Indian BioPharma companies
Building upon the workshop collaborations, a lecture by guest Prof. Anurag Rathore from the Indian Institute of Technology Delhi was organized. Prof. Rathore is a well-regarded thought leader in the field of BioPharma and is renowned for using quality by design (QbD) principles in BioPharma related processes. His talk focused on the characterization of mAbs using different biophysical techniques and applying QbD principles for process enhancement during characterizations. Prof. Rathore expanded on the BioPharma landscape of India and the challenges faced by Indian BioPharma companies in development of biosimilars.
Agilent offers ongoing workshops, seminars, and learning opportunities
This latest Agilent collaboration with industry thought leaders and customers was very well received. Participants gained new insights and explored comprehensive workflow solutions from Agilent for the analysis of biomolecules. Ongoing Biopharma workshops will cover topics such as CE, CEMS, and troubleshooting instruments. To learn more about Agilent’s full line-up of LC, LC/MS, and Q-TOF solutions, as well as MassHunter software, contact an Agilent representative today.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – November 2016
All articles in this issue
-
 Agilent 7010B and 7000D QQQ GC/MS systems expand your lab in multiple dimensions
Agilent 7010B and 7000D QQQ GC/MS systems expand your lab in multiple dimensions -
 Fast, accurate analysis of SVOCs with Agilent 9000 Intuvo GC
Fast, accurate analysis of SVOCs with Agilent 9000 Intuvo GC -
 Integrated workflow simplifies automated calculation of antibody-drug conjugate (ADC) drug-to-antibody ratio (DAR)
Integrated workflow simplifies automated calculation of antibody-drug conjugate (ADC) drug-to-antibody ratio (DAR) -
 Tips & tricks:
Tips & tricks:
How to simplify troubleshooting in your GC lab using Agilent ADM Flow Meter -
 Agilent collaborates with BioPharma thought leaders in India to advance biosimilar characterization
Agilent collaborates with BioPharma thought leaders in India to advance biosimilar characterization -
 Achieve fast, high throughput size exclusion chromatography of mAbs and ADCs using Agilent AdvanceBio SEC columns
Achieve fast, high throughput size exclusion chromatography of mAbs and ADCs using Agilent AdvanceBio SEC columns -
 Ask the Expert: How can I analyze virtually any polymer, from paints to polyolefins?
Ask the Expert: How can I analyze virtually any polymer, from paints to polyolefins? -
 Agilent LC/MS/MS solutions provide effective identification and quantitation of contaminants in botanical drug supplements
Agilent LC/MS/MS solutions provide effective identification and quantitation of contaminants in botanical drug supplements -
 Agilent JetClean self-cleaning ion source means you clean less, analyze more
Agilent JetClean self-cleaning ion source means you clean less, analyze more -
 High-throughput N-linked glycan profiling of monoclonal antibodies using Agilent integrated solution
High-throughput N-linked glycan profiling of monoclonal antibodies using Agilent integrated solution
Figure 1

Biopharma workshop sessions were held at the Agilent Center of Excellence lab (left) and at the Market development Centre India (right).
Figure 2

Results from the demonstrations of some workflows during workshop: (A) Charge variant analysis; (B) Size exclusion analysis of ADC; (C) Reversed-phase LCMS analysis of mAb; and (D) Drug antibody ratio analysis using Agilent’s DAR calculator.