用于 OpenLAB CDS ChemStation 版软件的安全工作站
Secure Data Storage Solution for Standalone Workstations
Scaled to meet the needs of small laboratories, the Secure Workstation for OpenLAB CDS ChemStation Edition provides comprehensive access control, data security, audit trails, and e-signature features that facilitate US FDA 21 CFR Part 11 compliance.
Agilent takes compliance seriously. We are continuously adapting to the changing needs of our customers that must comply with regulatory guidelines. To ensure that our OpenLAB software products and services address your mission critical compliance needs, we have on staff a Compliance Officer who is a highly-skilled 25-year veteran of the pharmaceutical and medical device industries.
Scaled to meet the needs of small laboratories, the Secure Workstation for OpenLAB CDS ChemStation Edition provides comprehensive access control, data security, audit trails, and e-signature features that facilitate US FDA 21 CFR Part 11 compliance.
Agilent takes compliance seriously. We are continuously adapting to the changing needs of our customers that must comply with regulatory guidelines. To ensure that our OpenLAB software products and services address your mission critical compliance needs, we have on staff a Compliance Officer who is a highly-skilled 25-year veteran of the pharmaceutical and medical device industries.
Our compliance solution provides:
- Fast, easy, and efficient secure storage and retrieval of LC/MSD, LC, GC, CE and SFC data
- Supports regulatory compliance with US FDA 21 CFR Part 11
- Supports OpenLAB CDS users’ current application workflows
- Competitively priced compliance for 1-2 instruments
The Secure Workstation provides a compliance solution for chromatography and mass spectrometry data for up to two instruments.
Solutions for Larger Laboratories
Networked and Enterprise solutions enable compliance for a multi-user, multi-instrument system with central data storage. User workflows are the same - no retraining required. Learn more
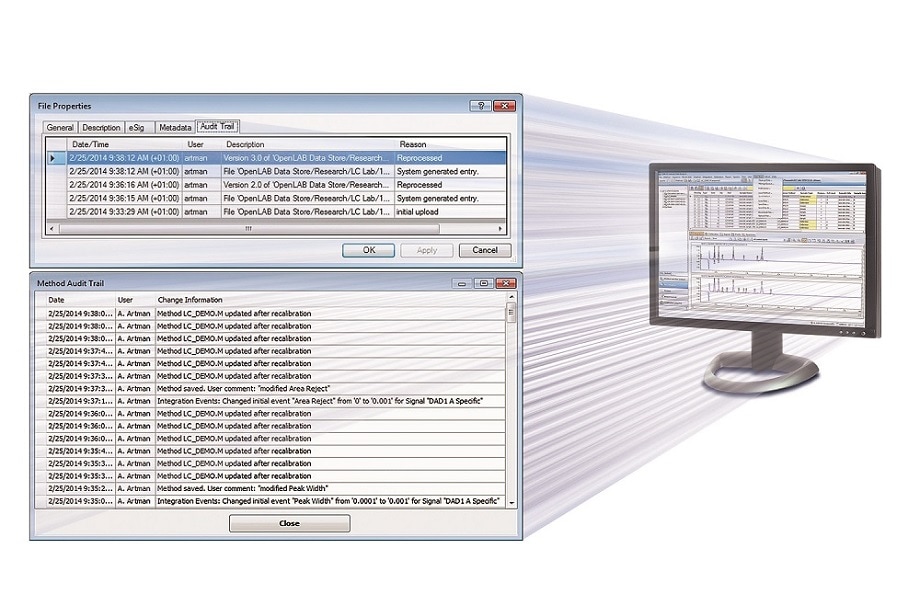
The Secure Workstation for OpenLAB CDS ChemStation Edition tracks all changes to records in the audit trail. A Method Audit Trail tracks changes to the method.