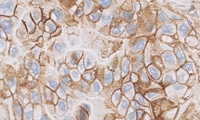
Tool - PD-L1 IHC 22C3 pharmDx NSCLC Interpretation Training Program

PD-L1 is a proven biomarker for patient response to KEYTRUDA in urothelial carcinoma
- Urothelial carcinoma is the fifth most common cancer in the United States and there is an urgent need for new effective treatments for patients
- For patients with advanced/metastatic urothelial carcinoma, the five-year survival rate is approximately 15%, and cancer-related mortality has not improved in the past 30 years
- Anti-PD-1 therapies are a promising new treatment class for many cancer types–the KEYNOTE-052 clinical trial studied the efficacy of KEYTRUDA, an anti-PD-1 monotherapy, at first-line in patients with locally advanced or metastatic urothelial carcinoma who were ineligible for cisplatin-containing chemotherapy
- IHC testing for PD-L1 expression levels enables identification of patients most likely to benefit from anti-PD-1 monotherapy
Proven in KEYNOTE-052 for PD-L1 results in urothelial carcinoma
- PD-L1 IHC 22C3 pharmDx was the only IHC assay used to assess PD-L1 expression in the KEYNOTE-052 clinical trial
- PD-L1 IHC 22C3 pharmDx offers the assurance of the only PD-L1 test FDA-approved as a companion diagnostic for KEYTRUDA

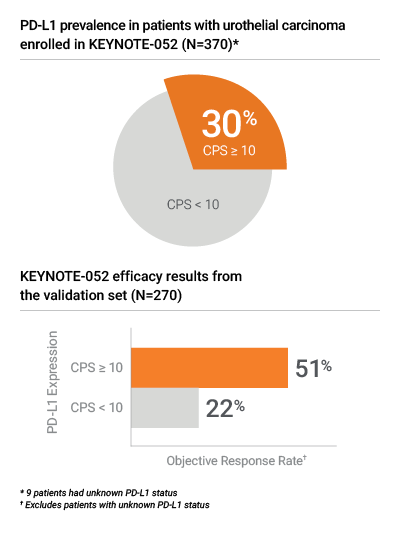
Early PD-L1 testing can provide critical information for physicians managing urothelial carcinoma patients who are ineligible for cisplatin-containing chemotherapy
- For patients ineligible for cisplatin-containing chemotherapy, there is a significant unmet need for treatment alternatives
- Age- and disease-associated comorbidities of urothelial carcinoma patients, including renal dysfunction and poor performance status, affect treatment choices and eligibility for standard cisplatin-containing chemotherapy
- In clinical practice, these comorbidities may be exacerbated by cisplatin-related toxicities
- There is no standard of care for patients ineligible for standard cisplatin-containing chemotherapy

* See the KEYTRUDA product label for specific clinical circumstances guiding PD-L1 testing


Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) and urothelial carcinoma tissues using EnVision FLEX visualization system on Autostainer Link 48.
Non-Small Cell Lung Cancer (NSCLC) PD-L1 protein expression in non-small cell lung cancer (NSCLC) is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. The specimen should be considered to have PD-L1 expression if TPS ≥ 1% and high PD-L1 expression if TPS ≥ 50%.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for expression cutoff values guiding therapy in specific clinical circumstances.
Urothelial Carcinoma PD-L1 protein expression is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100. The specimen should be considered to have PD-L1 expression if CPS ≥ 10.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying urothelial carcinoma patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for specific clinical circumstances guiding PD-L1 testing.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2018. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2018. 3. Gupta S, Gill D, Poole A, Agarwal N. Systemic immunotherapy for urothelial cancer: current trends and future directions. Cancers. 2017;9(15):1-14. 4. Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl. 2016;14(1):1-20. 5. Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet. 2017;18(11):1483-1492. 6. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):2432-2438.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.