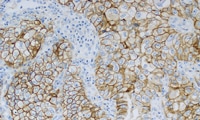
PD-L1 は非小細胞肺癌患者のキイトルーダに対する効果との相関が確認されています1,2
* KEYNOTE 臨床試験に関する詳細な情報はPD-L1 IHC 22C3 pharmDx「ダコ」の添付文書をご覧ください。
- 日本では肺癌の死亡率が癌の中で最も高いと報告されています6
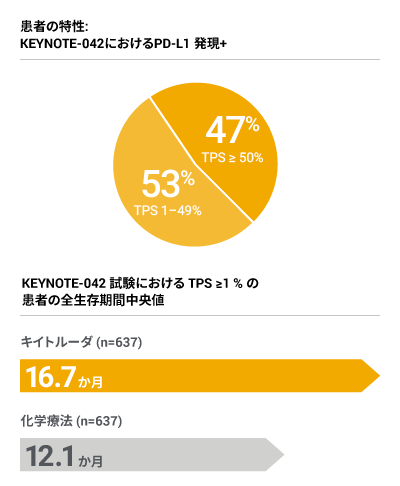
- KEYNOTE-042 臨床試験では、局所進行または転移性非小細胞肺癌で腫瘍の PD-L1 発現 (TPS ≥ 1 %) の患者を対象に抗 PD-1 単剤療法であるキイトルーダの有効性が検証されました*
-
IHC 検査で PD-L1 発現率を測定することによって、抗 PD-1 単剤療法が有効であると思われる患者を特定することができます
KEYNOTE-042 臨床試験にて非小細胞肺癌における PD-L1 発現率を測定1,2
PD-L1 IHC 22C3 pharmDx「ダコ」は、KEYNOTE-042 臨床試験で PD-L1 発現評価に使用され、キイトルーダ治療に適した患者を確認することができるコンパニオン診断薬です

†≥ PD-L1 発現 (TPS ≥ 1 %) の認められたがん患者数に基づきパーセントを計算。PD-L1 非発現がん患者は計算対象外。
使用目的
体外診断用
PD-L1 IHC 22C3 pharmDx「ダコ」は、抗 PD-L1 マウスモノクローナル抗体 (Clone 22C3) を用いた免疫組織化学染色法を原理としたアッセイキットです。ダコ Autostainer Link 48 (IHC 自動染色装置) を用いて、ホルマリン固定パラフィン包埋 (FFPE) 非小細胞肺癌 (NSCLC) 組織中の PD-L1 発現率を測定するためのものです。
非小細胞肺癌中の PD-L1 発現率は、任意の強度で部分的または完全な細胞膜染色を示す陽性腫瘍細胞の割合である Tumor Proportion Score (TPS) を用いて決定します。
| 適応腫瘍† | PD-L1 発現 | 使用目的 |
| NSCLC | TPS ≥ 1 % | がん組織、細胞中のPD-L1発現率の測定 (非小細胞肺癌患者におけるペムブロリズマブ (遺伝子組換え) の適切な投与を行うための補助に用いる。) |
† 詳細な染色の解釈については、添付文書の判定法と NSCLC に対応した「PD-L1 IHC 22C3 pharmDx「ダコ」染色結果判定マニュアル」を参照してください。
キイトルーダは、Merck & Co., Inc の子会社である Merck Sharp & Dohme Corp. の登録商標です
参考文献:1.PD-L1 IHC 22C3 pharmDx「ダコ」添付文書 アジレント・テクノロジー株式会社; 2018 2.キイトルーダ添付文書 MSD 株式会社; 2018 3.Wang F, Mishina S, Takai S, et al.Systemic treatment patterns with advanced or recurrent non-small cell lung cancer in Japan: A retrospective hospital administrative database study.Clinical Therapeutics.2017;39(6):1146-1160 4.The Japan Lung Cancer Society.Guidelines for clinical practice of lung cancer: Stage IV non-small cell lung cancer. https://www.haigan.gr.jp/guideline/2017/1/2/170102060100.html#j_1_2_6-0_01.Published 2017.Accessed November 5, 2018. 5.Roach C, Zhang N, Corigliano E, et al.Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer.Appl Immunohistochem Mol Morphol.2016;24:392-397. 6.National Cancer Center, Japan. Projected cancer statistics, 2018.(https://ganjoho.jp/en/public/statistics/short_pred.html)