Access Agilent eNewsletter June 2016
Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products
Andreas Tei, Agilent Global Pharma Segment Manager
In November 2015 and in January 2016 we published two articles in Access Agilent that described a general workflow to analyze extractables and leachables (E&Ls) as impurities in pharmaceutical products. Volatile and semi-volatile E&Ls were recently analyzed by headspace and multimode injection GC/MS in various pharmaceutical products. These analyses were described in Agilent Application Notes 5991-5616EN, 5991-5605EN, 5991-5632EN, 5991-6142EN. This article continues the series and describes high-resolution accurate-mass GC/MS identification of extractable and leachable compounds from an ophthalmic drug product.
Why high-resolution GC/MS?
Confirmation of compounds based on comparison of their nominal-mass electron ionization (EI) mass spectra with spectra data in commercially available database poses a high risk of uncertainty and provides only a tentative identification. To confirm the identities, you must analyze reference compounds under the same chromatographic conditions and compare the spectra and retention times of the unknowns with those of the reference compounds.
High-resolution mass spectrometry combined with collision-induced dissociation (CID) is a powerful technique for a streamlined and automated identification process. The presence of a compound is confirmed by calculating the chemical formula of its molecular ion and of its most abundant fragment ions. Once a compound has been identified by its exact mass data, the information will be saved in Personal Compound Databases (PCD) and used for a confident and automated identification process in further analytical tasks.
To demonstrate, we examine an automated workflow that uses Agilent Mass Profiler Professional data mining software to detect and confirm—based on accurate-mass data—the presence of compounds in complex matrices. The method uses chemical ionization—a soft ionization technique—followed by collision-induced dissociation to produce fragment ions.
Sophisticated workflow delivers reliable answers
We investigated an ophthalmic drug product to determine the presence of impurities that originated from the polymer material of the container closure system. Figure 1 shows the workflow that included the following steps:
- Data acquisition in high-resolution electron ionization (HR-EI) mode
- Automated library match (nominal spectral library NIST 14)
- Differential analysis of blanks and extracts with Agilent Mass Profiler Professional software
- Confirmation of tentatively identified compounds using high-resolution chemical ionization (HR-CI) MS and HR-CI MS/MS data
- Semi-quantitation
The drug container was extracted with an organic solvent and the sample was analyzed with an Agilent 7200 GC/Q-TOF using electron ionization (EI). A solvent blank sample was also investigated to determine the chemical background contamination.
In addition, we extracted the aqueous drug formulation with an organic solvent. Data analysis used a two-step process. In the first step, the acquired mass spectra were automatically compared with nominal mass spectra in the NIST 14 library. A list of extracted compounds for each acquired chromatogram was generated. This compound list was then subjected to a differential analysis process to subtract the chemical background noise and to find common compounds in both samples. Agilent Mass Profiler Professional software was used for this data mining process.
We obtained a reduced number of tentatively identified compounds that were common in the container extract and in the drug formulation. Chemical ionization was combined with collision-induced dissociation (CID) and accurate-mass spectra to confirm the tentatively identified compounds. The compounds we identified based on both EI and CI accurate-mass data were saved in a Personal Compound Database and Library (PCDL), to enable a streamlined compound confirmation process in further analytical tasks.
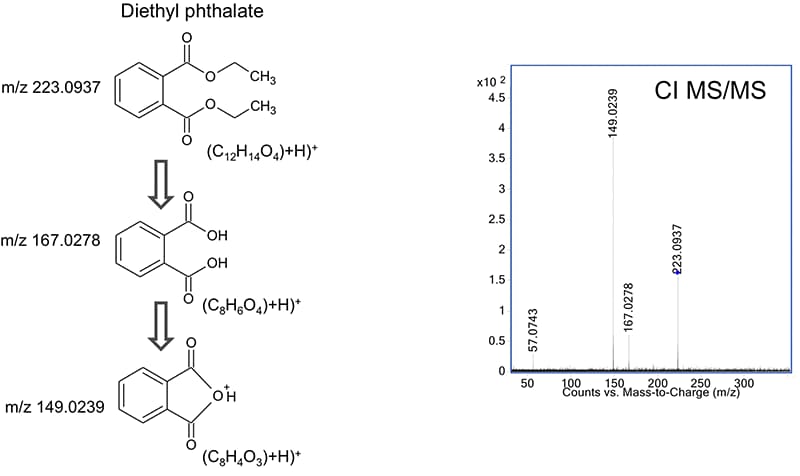
Figure 2. This chemical ionization high-resolution MS/MS spectrum confirmed the presence of diethyl phthalate.
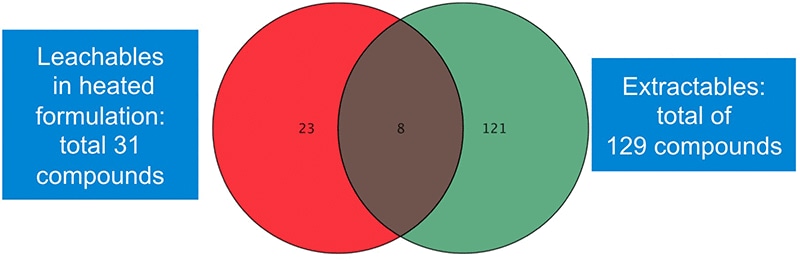
Figure 3. Data mining with Agilent Mass Profiler Professional revealed more than 150 leachable and extractable compounds.
Collision induced dissociation eliminates ambiguity
Tentatively identified compounds that show extensive source fragmentation in EI mode can be confirmed by chemical ionization combined with collision-induced dissociation. As shown in Figure 2, high-resolution accurate-mass MS/MS data confirm the structure based on its fragmentation pattern. To speed later analyses, you can store CI MS/MS spectra in a PCDL to build a custom library.
Our last step was semi-quantitation of the identified compounds. We used a surrogate mix that contained various reference compounds as an external standard. We used this standard to estimate the concentrations of the found compounds and to compare the concentrations with the analytical evaluation thresholds (AETs).
In this example, none of the detected compounds were close to a concentration of 1 ppm in the drug formulation, so a more accurate quantitation using matched reference compounds was not required.
The Venn diagram in Figure 3 shows results of a differential analysis data mining process using Agilent Mass Profiler Professional software.
Table 1 lists the eight common compounds that were found in the extract of the container and in the liquid drug formulation.
| Retention time | Extractable and leachable compounds | Fold change in extractable |
|---|---|---|
| 8.75 | Octane, 3,5-dimethyl | UP |
| 15.16 | Benzene, 1,3-bis(1,1-dimethylethyl)- | UP |
| 15.75 | Dodecane, 4,6-dimethyl | UP |
| 16.19 | Tridecane | UP |
| 16.20 | Nonadecane | UP |
| 16.87 | Cyclohexasiloxane, dodecamethyl- | UP |
| 19.92 | Sulfurous acid, pentyl undecyl ester | UP |
| 20.53 | Cycloheptasiloxane, tetradecamethyl- | UP |
Table 1. Common compounds that were found after differential analysis.
Powerful tools accelerate E&L analyses
An Agilent 7200 GC/Q-TOF is an ideal instrument for high-resolution accurate-mass qualitative screening and identification of extractable and leachable compounds from drug products. To aid compound identification, you can match the EI spectra with the NIST 14.0 library. Agilent Mass Profiler Professional software enables differential analysis of sample sets, where Venn diagrams help determine unique and common compounds across sample groups. Accurate-mass CI MS/MS spectra confirm tentative hits and expand the list of identified compounds. You can store these spectra in a PCDL to build a custom library that accelerates future analyses.
For more information about these highly informative analyses, explore Application Notes 5991-6688EN and 5991-6348EN.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
-
 New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity
New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity -
 New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency
New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency -
 Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows
Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows -
 Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD
Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD -
 Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs
Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs -
 Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology
Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology -
 Excellent inertness for analysis of challenging polar compounds
Excellent inertness for analysis of challenging polar compounds -
 Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products
Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products -
 Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds
Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds -
 Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
A real-life application
Figure 1
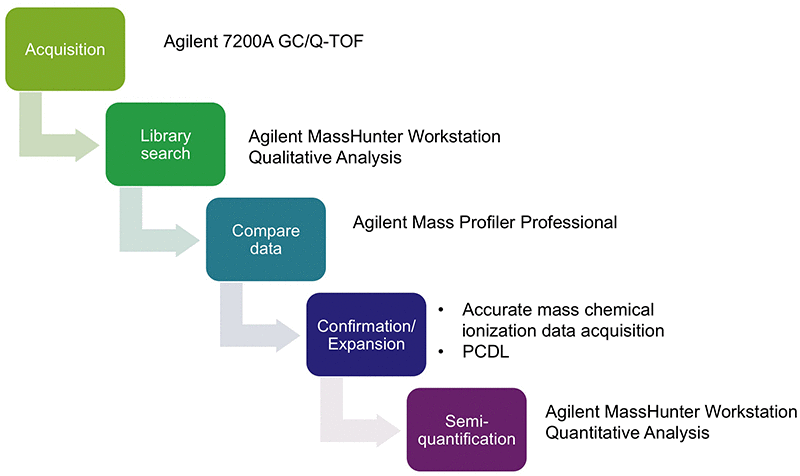
Reliable workflow for analysis of impurities from a pharmaceutical container.
Figure 2

This chemical ionization high-resolution MS/MS spectrum confirmed the presence of diethyl phthalate.
Figure 3

Data mining with Agilent Mass Profiler Professional revealed more than 150 leachable and extractable compounds.