Access Agilent eNewsletter November 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Tip: Keep pharmaceutical products free from extractables and leachables
By Andreas Tei
Agilent Pharma Segment Manager – Small Molecules
The study of extractables and leachables (E&L) as impurities in drug products has become increasingly important. There have been many recalls linked to potentially toxic materials originating from drug containers or parts of the packaging. E&Ls comprise a large variety of compounds, from typical plasticizers as potential endocrine disruptors to genotoxic compounds, such as nitrosamines, polyaromatic hydrocarbons, or toxic elements. E&Ls are detrimental to the integrity of the drug product itself. In addition, the effectiveness of biosimilars could be degraded after contamination with reactive organic compounds or with heavy metal ions.
What’s the difference?
Extractables are compounds that have been extracted from drug containers as a worst-case scenario under drastic conditions. Leachables are usually a subset of extractables found in the drug formulation itself. So-called migrants are compounds that have migrated through the primary container and are also found in the drug formulation. They often originate from the inks on labels or from the environment due to compromised storage conditions and are not a subset of extractables. Figure 1 summarizes the relationships between the different types of contaminants.
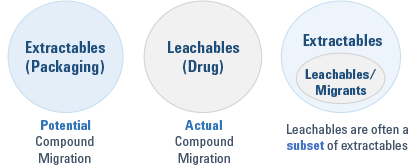
Figure 1. Different types of contaminants found in pharmaceutical products.
Why worry about E&Ls?
As FDA data show, the last two years have seen almost as many recalls (2,061) as the previous nine years combined (2,217), and that’s only counting the first seven months of 2014. Defects included the presence of particulate matter, metal, or glass, and also chemical contamination. For example, batches of a gastro-resistant drug were recalled last year because of complaints of unpleasant ‘fishy’ or ‘sweaty’ odors. The root cause was aluminum foil used in the packaging. Although there was no impact on the efficacy or quality of the drug, there was an economic and reputational cost to the drug manufacturer, and to the supplier of the aluminum packaging.
Defining the risks
The U.S. Food and Drug Administration (FDA) has ranked the different drug formulations (liquid or solid) in several risk categories. These depend on the route of administration and the likelihood of interaction between the drug product and its containment. The manufacturer must calculate an analytical evaluation threshold for each compound based on the number of doses and the amount of incorporated drug per day.
An FDA-related organization, the Product Quality Research Institute, has provided definitions to help identify the limits of quantification. Any leachable identified at or above 5.0 µg/day should be reported for a potential toxicological assessment. A safety concern threshold below 0.15 µg/day was defined as the threshold of a leachable compound that would present negligible safety concerns from possible carcinogenic to noncarcinogenic toxic effects.
Analytical approach to E&L analysis
The workflow of sample preparation and the method of detection employed for the determination of E&Ls must be chosen with great care since there is no universal detector that will analyze all possible E&Ls. Usually, employing complementary and orthogonal analytical techniques is the best screening approach for determining E&Ls arising from the packaging. Agilent offers a wide array of complementary solutions that are very effective in E&L analysis. For example…
- Agilent 7697A Headspace Sampler can be paired with the Agilent 5977A Series GC/MSD system for the detection of volatile organic compounds and high migration potential species, such as inks, adhesives, and processing solvents.
- Agilent 7890B GC, Agilent 7010 Triple Quadrupole GC/MS, and Agilent 7200 GC/Q-TOF can be combined to detect semivolatile organic compounds, residual monomers, antioxidant, plasticizers, antistatic agents, clarifying agents, preservatives, PAHs, and slip agents.
- Agilent 6545 Q-TOF LC/MS or LC/UV can be utilized to identify and measure nonvolatile organic compounds, large oligomers, large antioxidants, and thermally labile compounds.
- Agilent 7900 ICP-MS and 5100 ICP-OES are a good detection "team" for metals from aluminum canisters or glass bottles.
 Enlarge
Enlarge
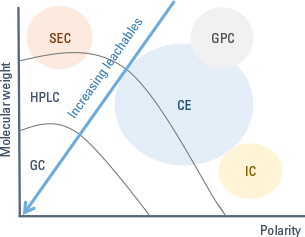
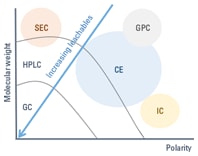
Figure 2. Select the analytical technique according to the polarity and molecular weight of the analytes (CE – capillary electrophoresis, GPC – gel permeation chromatography, SEC – size exclusion chromatography, IC – ion chromatography).
The technique used depends on the polarity and molecular weight of the compounds of interest (Figure 2).
Agilent solutions for E&L analysis
Cutting edge solutions from Agilent, covering the whole range of analytical platforms for screening extractables in drug product packaging and measuring leachables in drug formulations, are readily available to assist you with your E&L analysis. Agilent understands the challenge of interpreting data from these complex analyses. Take a moment and explore our huge selection of E&L solutions, including data-mining technologies to aid sample comparison, database-driven approaches to quickly identify known compounds, and structure identification tools to help identify unknowns.
Want to learn more?
Agilent has published widely on E&L analysis, with more articles on the way. These reading materials include a new white paper ICH and USP methods for elemental analysis using ICP-MS and ICP-OES, and Application Notes on GC/MSD analysis of plastic intravenous bag sets, transdermal patches, and generic drug formulations. Upcoming publications will feature an investigation of a drug container closure system using high-resolution GC/MS and LC/MS and an exploration of data mining for E&L screening. Look to your next issue of Access Agilent for discussion of these applications in greater detail.
>> Update My Profile | Subscribe to Access Agilent | Article Directory