Access Agilent eNewsletter March 2016

Quick, accurate screening for suspect environmental contaminants with Agilent solutions
Lakshmi Krishnan
Agilent Global Marketing Manager—Environmental
Craig Marvin
Agilent Environmental Segment Marketing Manager
Exposure to emerging contaminants in air, water, and soil may present significant risks to the global community. These concerns are driving researchers to identify the scope and origin of such pollutants, leading to a demand for innovative analytical techniques for nontarget, or suspect screening.
There are many standard methods for routine analysis of environmental pollutants in water and other matrices. However, as the range of pollutants increases and regulators demand ever-lower limits of detection, it is important to take advantage of improved technologies to maintain the competitive edge of your lab.
To facilitate accurate identification of targets and suspect chemical contaminants, research labs rely increasingly on proven, end-to-end solutions for screening suspect contaminants in environmental and biological matrices. For example, Agilent GC/Q-TOF systems and LC/Q-TOF instruments provide separation and spectral resolution to help positively identify chemical contaminants and avoid false negatives. Data mining tools such as Agilent MassHunter, MassProfiler Professional, and extensive Agilent Personal Compound Database Libraries deliver fast and efficient data evaluation across systems.
Identify toxins from algal blooms in fresh water with Agilent LC/MS/MS
The occurrence of cyanobacterial toxins in Canadian fresh waters is a serious environmental and public health concern. Microcystins are a class of common cyanobacterial toxins found in Canadian lakes, which are potent inhibitors of eukaryotic protein phosphatases and powerful hepatoxins.
The Alberta Centre for Toxicology (ACFT) and Vogon Laboratory Services developed a confirmation method for microcystins using Agilent 1290 Infinity LC and Agilent 6460 Triple Quadrupole LC/MS, with a different retention time pattern from the reference ACFT method. Accurate mass determination on an Agilent 6540 Q-TOF LC/MS was then used to tentatively identify an unknown peak as desmethylated microcystin HtyR. An Agilent Poroshell 120 SB-C18 LC column was used for the separation.
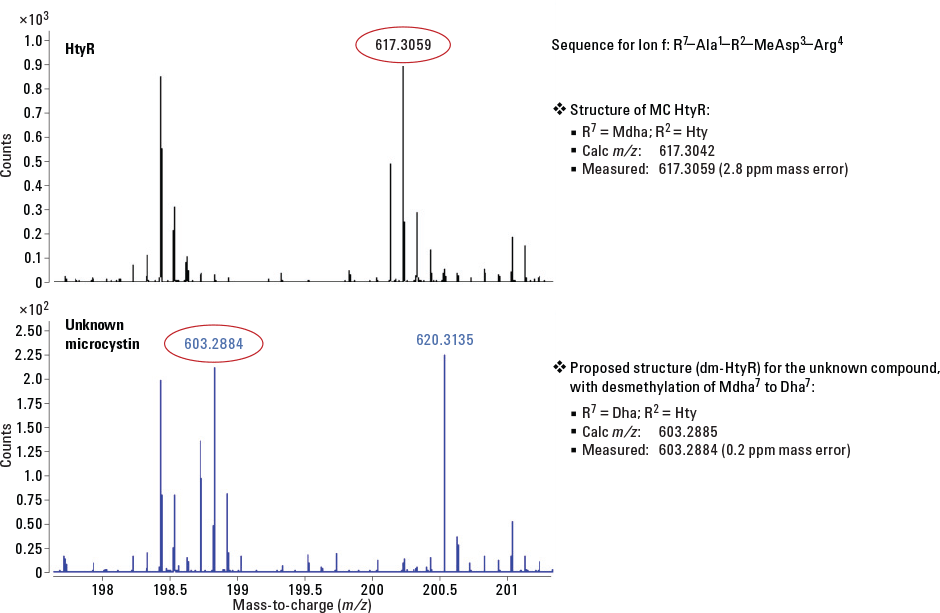
Figure 1. Q-TOF spectra of an ion for HtyR and the unknown microcystin. Analysis of the HtyR standard gave the correct accurate mass.
Analysis by Q-TOF of an ion in the unknown microcystin revealed accurate masses that confirmed the presence of Dha at position 7 and Hty at position 2, consistent with the identification of the unknown as dm-HtyR. Figure 1 shows how the accurate mass for the ion in HtyR (upper) matched the calculated mass for the ion containing Hty at position 7 and Mdha at position R3. In contrast, the mass observed for the ion in the unknown microcystin matched the calculated mass for the ion containing Hty at position 7 and Dha at position R3.
The accurate mass capabilities of the Agilent 6540 Q-TOF LC/MS was used to tentatively identify other microcystins, based only on the mass of their H+ adducts. Using Agilent MassHunter PCDL Manager software, a personal compound database (PCD) was built. The software lets you create and edit a customizable PCD, including compounds, accurate-mass, and retention time information. An advantage of accurate-mass scan data is the ability to retrospectively search acquired data for new compounds using such a database.
The WHO list of microcystins was then used to enter the formulas for 52 microcystins into a PCD, which generated accurate masses for the H+ adducts. Next, the Find by Formula tool in Agilent MassHunter software was used to search for these accurate masses in the sample TIC data file. Seven unknowns were tentatively identified in samples from Lake A and Lake B, in addition to desmethyl HtyR.
Learn more about these results in Agilent publication 5991-4444EN.
Screen semivolatiles in airborne particulates with Agilent GC/MS/MS
Screening semivolatile organic compounds (SVOCs) has become a more demanding and complex task that requires enhanced selectivity, sensitivity, and a nontargeted workflow for data analysis. An accurate-mass approach using Q-TOF offers more reliable identification, and allows for a virtually unlimited number of semivolatiles to be screened simultaneously.
Collaborators at Fudan University and Agilent Shanghai developed a nontargeted screening workflow for SVOCs absorbed in aerosol particles. The system comprised a high-resolution Agilent 7890B GC coupled to an Agilent 7200A Q-TOF, with two Agilent J&W HP-5ms Ultra Inert GC columns.
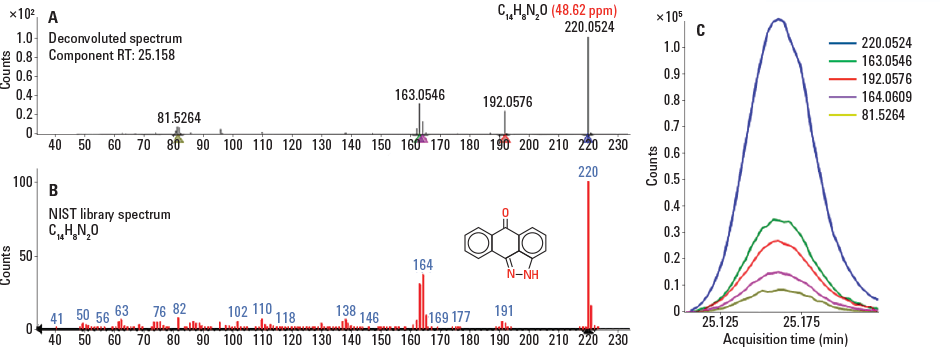
Figure 2. Comparison of mass spectra between an unknown compound and a tentative NIST library match (A, B).
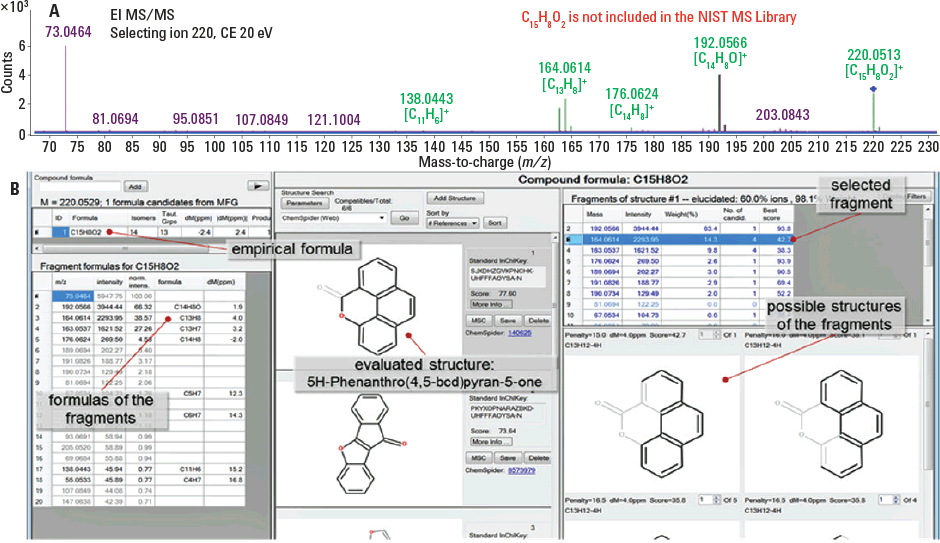
Figure 3. Empirical formulas generated from an MS/MS spectrum using Formula Generator (A), and structure elucidation of a semivolatile with an empirical formula of C15H8O2 using MSC software (B).
For this analysis, compound hits were obtained by using deconvoluted mass spectra that were searched against a NIST nominal-mass spectral library. The accurate mass of molecular ion or fragment ions was used to confirm compound formulas. As an added advantage, the Agilent 7200A Q-TOF system can be operated in MS/MS mode to investigate structures of unknowns.
Chromatographic peak deconvolution discovered unknown compounds (Figure 2). The closest match for this spectrum in the NIST library was anthra[1,9-cd]pyrazol-6(2H)-one. However, this tentative match could be readily rejected based on mass accuracy alone, since the error on the molecular ion was 48.62 ppm. This highlights the advantage of accurate-mass data obtained from a Q-TOF versus a unit mass instrument.
Figure 3 shows the workflow using MS/MS mode with accurate-mass fragments to propose the structure of the unknown compound. The Agilent MassHunter Formula Generator tool was used to assign an accurate empirical formula to the molecular and major fragment ions (each fragment ion is ranked based on mass error corresponding to the proposed formula, along with a penalty based on how many bonds needed to be broken)The spectrum was then imported into MassHunter Molecular Structure Correlator (MSC). Next, MSC searched the ChemSpider database to find all possible structural isomers. Although this type of confirmation is not completely unambiguous, it provides extra validation for this tentatively identified O-PAH.
Explore Agilent publication 5991-5899EN for further details of this comprehensive analysis.
Agilent offers a wide range of solutions for detecting environmental contaminants
Look to Agilent to provide your busy lab with leading-edge liquid chromatography and mass spectrometry analytical tools, as well as data mining tools to meet all of your environmental contaminant detection needs. These solutions deliver superior data quality, high spectral resolution, and speedy results, while greatly reducing the possibility for false negatives. Contact an Agilent Representative today to find out more about solutions for suspect screening.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – March 2016
All articles in this issue
 Evolution of multiple heart-cutting 2D-LC: Agilent 1290 Infinity II provides high-resolution sampling and easy quantitation
Evolution of multiple heart-cutting 2D-LC: Agilent 1290 Infinity II provides high-resolution sampling and easy quantitation Tip: How to achieve efficient, effortless LC method transfer to the Agilent 1290 Infinity II LC System
Tip: How to achieve efficient, effortless LC method transfer to the Agilent 1290 Infinity II LC System Agilent 1290 Infinity II RID delivers high resolution and rapid polymer characterization
Agilent 1290 Infinity II RID delivers high resolution and rapid polymer characterization Dual-needle option for LC autosamplers provides improved speed, flexibility, and accuracy
Dual-needle option for LC autosamplers provides improved speed, flexibility, and accuracy Multiple heart-cutting 2D-LC/MS method for reproducible resolution of chiral drug metabolites
Multiple heart-cutting 2D-LC/MS method for reproducible resolution of chiral drug metabolites Quick, accurate screening for suspect environmental contaminants with Agilent solutions
Quick, accurate screening for suspect environmental contaminants with Agilent solutions
Figure 1

Q-TOF spectra of an ion for HtyR and the unknown microcystin. Analysis of the HtyR standard gave the correct accurate mass.
Figure 2

Comparison of mass spectra between an unknown compound and a tentative NIST library match (A, B).
Figure 3

Empirical formulas generated from an MS/MS spectrum using Formula Generator (A), and structure elucidation of a semivolatile with an empirical formula of C15H8O2 using MSC software (B).