Access Agilent eNewsletter July 2016

Structural elucidation of in-process impurities using high resolution LC/MS/MS
Syed Salman Lateef, Agilent CrossLab Group
With the paramount importance of consumer safety in mind, the characterization of impurities in drug products has become a crucial regulatory requirement in the pharmaceutical drug development process. Identification of impurities and their structural elucidation is greatly facilitated when using high resolution accurate mass LC/MS together with comprehensive software tools for data processing and structural characterization. The in-process impurities are often structurally related to process ingredients or intermediates. Therefore, a direct comparison of accurate mass MS, MS/MS, and pseudo MS3 of unknowns with known standards assists in structural elucidation. In this article, we review a study using a four-step LC/MS workflow for successful elucidation of impurities in drug products.
Powerful Agilent MSC software delivers successful data interpretation
In this study, available amlodipine impurity standards were used to create an accurate mass MS database together with MS/MS and pseudo MS3 spectral libraries using the Agilent Personal Compound and Database Library (PCDL) software. The accurate mass data for the unknown in-process impurities were then searched against the known libraries to identify common substructures. Elemental compositions and associated structures for the impurities could then be proposed. Also used in this analysis was Agilent Molecular Structure Correlator (MSC), an intelligent software tool. MSC software correlates accurate mass and elemental formula of MS/MS fragments with possible in silico fragment ions from proposed structures. Determination of structures of fragment ions and elucidation of fragmentation pathways were achieved using the MSC software.
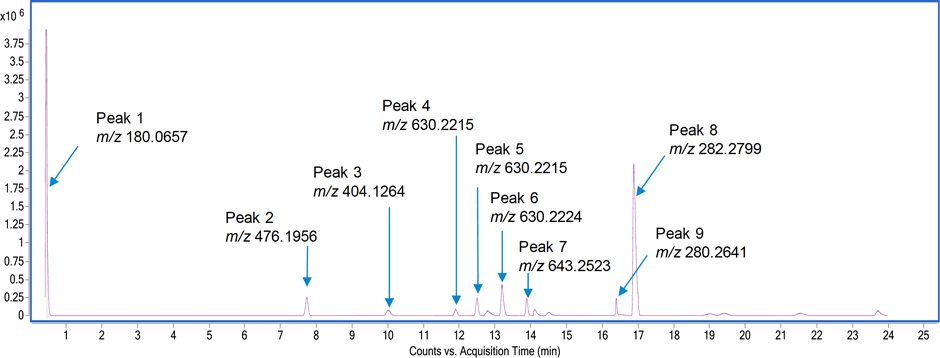
Figure 1. TIC (background subtracted) of the in-process samples showing nine compounds and their measured accurate masses.
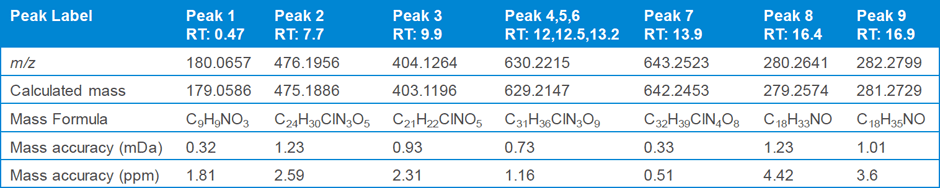
Table 1. Nine compounds detected from the unknown impurity samples and their calculated mass errors.
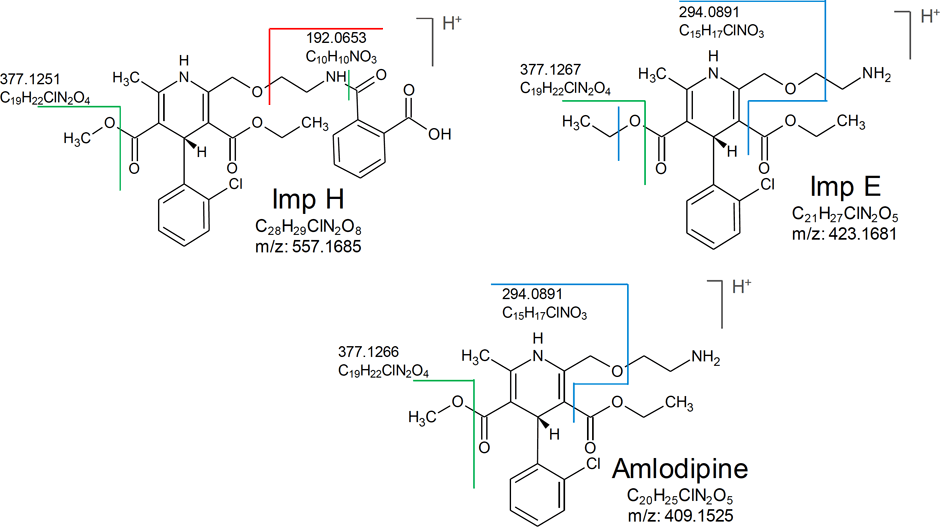
Figure 2. Proposed structures and fragmentation sites for known impurities.
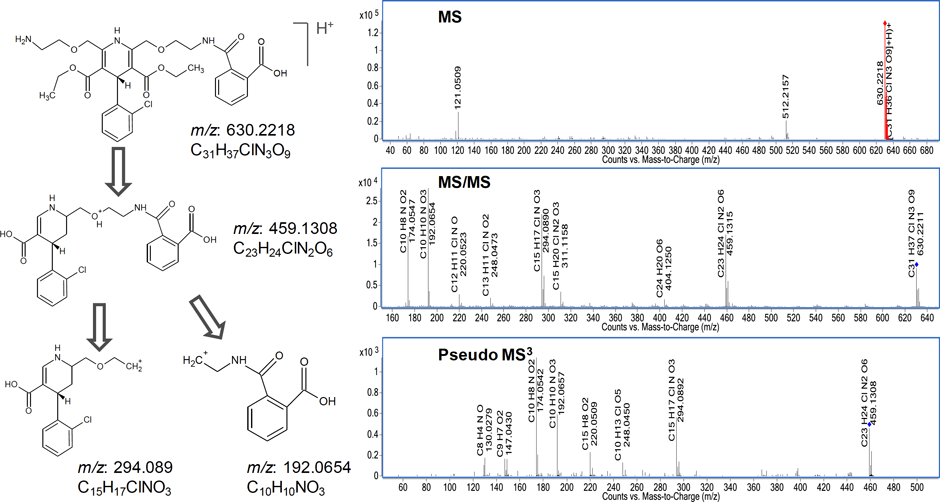
Figure 3. Proposed structure and fragmentation pathways of the unknown ion at m/z 630.2218. The pseudo MS3 of m/z 459.1308 revealed that it is an intermediary for the fragment ion at m/z 294.089 and m/z 192.0654
An effective four-step LC/MS workflow for unknown structure elucidation
This analysis utilized a four-step LC/MS workflow that delivered successful elucidation of amlodipine impurities.
In the first step, MS, MS/MS, Pseudo MS3, two injections per sample with high and low fragmentor voltages were performed. This was followed by data dependent MS/MS acquisition at three different collision energies.
At step two, data analysis step, the unknown sample analysis in MS mode revealed nine components (Figure 1) using the molecular feature extraction algorithm. Table 1 gives the formula of each of these compounds, along with the mass error.
At step three, fragmentation pattern analysis is performed. This is accomplished with a fragment search of unknowns using the PCDL software. Figure 2 shows a comparison of fragments—similarities, losses, and differences between known and unknowns is achieved.
In this example, a custom spectral library of known impurity standards was created using the Agilent Personal Compound and Database Library (PCDL) software. By using the fragment search feature of PCDL and fragmentation site comparison with known impurities, the structure of unknown impurities was proposed and verified. The MS/MS fragment at m/z 294.0880, of Peak 4 was found in Impurity E, while the ion at m/z 192.0653 was found in Impurity H [1]. In addition, the pseudo MS3 spectra of the ion at m/z 294.0880 showed a similar fragmentation pattern to the ion at m/z 294.0880 in Impurity H and E.
For the fourth step, we look at a structure proposal. Using MSC and ACD software, we conduct verification of the structural fragmentation pathway.
Figure 3 illustrates the fragmentation pathway for the ion at m/z 630.2215. The proposed structures and fragmentation were confirmed using the ACD software. The proposed structure was fragmented in silico by selecting ESI fragmentation. The results yielded the fragments m/z 294.089 and m/z 192.0654, and are consistent with expected fragmentation mechanisms.
Fast, effective separation of impurities in pharmaceuticals with Agilent LC/MS/MS
Our example described the structure elucidation of amlodipine impurities. As a result of this analysis, nine impurity compounds were separated and identified. These have a range of different chemical structures, and were formed during the commercial synthesis process. Available standards and in-process impurity samples were analyzed by high-resolution accurate mass LC/MS and LC/MS/MS using an Agilent 1290 Infinity LC system, Q-TOF-MS, and pseudo MS modes. The fragmentation patterns of known compounds were determined and stored in the Agilent MassHunter PCDL software to enable fragment searching within the custom database. The fragmentation sites and pathways of known standard compounds were determined using Molecular Structure Correlator software to confirm the cleavage sites and to propose structures and substructures for the unknowns. Thus, the combination of intelligent software and high-resolution accurate mass data enabled the structures of several impurities to be determined.
Full details of this study are readily available in Agilent Application Note 5991-6411EN.
Agilent provides a wide range of LC and LC/MS solutions
Agilent offers today’s busy laboratory managers a huge selection of Liquid Chromatography and LC/MS solutions. From the Agilent 1290 Infinity LC system to LC columns, mass spectrometry, Q-TOF-MS, and PCDL software, Agilent solutions deliver increased efficiencies, higher throughputs, cost-savings, and ease of use.
Reference
- D. Udawant, et al. “Identification and Structural Elucidation of Amlodipine Impurities Using High Resolution LC/MS and LC/MS/MS.” Agilent Application Note 5991-6411EN.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application
Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds
Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds Tip: Using enzymes in dissolution testing of gelatin capsules
Tip: Using enzymes in dissolution testing of gelatin capsules InfinityLab Poroshell 120 columns maximize LC workflow efficiency
InfinityLab Poroshell 120 columns maximize LC workflow efficiency Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research
Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research New LC/TQ database and method for central carbon metabolites
New LC/TQ database and method for central carbon metabolites Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication
Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns
Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns Structural elucidation of in-process impurities using high resolution LC/MS/MS
Structural elucidation of in-process impurities using high resolution LC/MS/MS Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS
Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Figure 1

TIC (background subtracted) of the in-process samples showing nine compounds and their measured accurate masses.
Table 1

Nine compounds detected from the unknown impurity samples and their calculated mass errors.
Figure 2

Proposed structures and fragmentation sites for known impurities.
Figure 3

Proposed structure and fragmentation pathways of the unknown ion at m/z 630.2218. The pseudo MS3 of m/z 459.1308 revealed that it is an intermediary for the fragment ion at m/z 294.089 and m/z 192.0654