Access Agilent eNewsletter May 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Tips: Optimize glycan mapping with AdvanceBio workflow solutions from Agilent
By Andrew Coffey
Agilent Applications Chemist
Monoclonal antibodies (mAbs) dominate the biotherapeutic market, with numerous products designed to provide treatments for cancer or immune diseases such as Crohn’s disease, rheumatoid arthritis, and asthma. Many hundreds of compounds are undergoing clinical trials. However, most of the approved mAbs are human or humanized. They are prepared using mammalian cell culture, engineered to create a protein sequence comparable to the human variant. Once expressed, the antibody undergoes glycosylation, a post-translational modification. The Agilent AdvanceBio Glycan Mapping workflow provides a timesaving, optimized method to generate glycosylation profiles for these important biotherapeutics.
The glycan structure attached to the protein during glycosylation is particularly important because it affects biological activity. The immune response relies on the interaction between the Fc (fragment crystallisable) region of the antibody and receptors on the immune cell surface. Interactions may also occur with other molecules involved in antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). The actual structure can affect the level of interaction.
 Enlarge
Enlarge
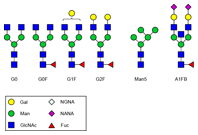
Figure 1. Common N-glycans from therapeutic monoclonal antibodies. The Agilent solution makes it easy to monitor production of these individual structures.
Efficiently generate glycosylation profiles with a single-vendor solution
For bioengineered therapeutic mAbs, the glycans are primarily attached to the asparagine Asn297 residue in the conserved Fc region of the antibody. The glycan that is initially attached to the protein is then enzymatically modified to generate a wide variety of individual structures (Figure 1). Production of a controlled glycan profile therefore remains a challenge, both during development and subsequently during manufacture. You must continually monitor this production.
Identification of individual glycan structures generally relies on MS-based techniques. However, you can achieve profiling and quantification with HPLC.
The Agilent AdvanceBio Glycan Mapping workflow provides the complete solution for:
- Cleavage of N-glycans
- Labelling of glycans
- Separation by HILIC (hydrophilic interaction liquid chromatography)
- Use of reference standards for method optimization
This workflow enables you to generate glycosylation profiles in an efficient, robust, and reproducible manner with a single-vendor solution.
 Enlarge
Enlarge
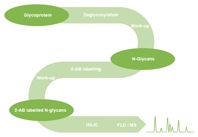
Figure 2. Agilent provides a complete solution to accelerate this typical workflow for glycan analysis.
Glycan mapping (Figure 2) is ideally suited to HILIC separation. The N-glycans must be cleaved from the protein using the enzyme PNGase-F. You achieve this cleavage by incubation for three hours at 37 °C. The cleaved N-glycans must then be purified from the cleavage solution by solid phase extraction (SPE). To increase sensitivity and eliminate anomerization of the cleaved glycans, you label them with industry-standard 2-aminobenzamide (2-AB). The labelling reaction requires three hours at 65 °C. The 2-AB labelled glycans are then purified using SPE cartridges.
Advanced columns for glycan mapping
Agilent offers two types of HILIC glycan mapping columns: 1.8 µm totally porous and 2.7 µm superficially porous. The mechanism of HILIC separation involves partitioning into an aqueous-rich environment on the surface of the particles. The 1.8 µm material is designed for UHPLC applications and requires an Agilent 1290 Infinity LC. The 2.7 µm material is available in a wider selection of column dimensions and can be used on an Agilent 1260 Infinity LC or even older instruments with pressure limits of 400 bar. The limited quantities of 2-AB labelled glycans in your sample and the small injection volumes will require sensitive fluorescence detection.
All HILIC phases are operated from high organic to lower organic mobile phase. The organic mobile phase is normally acetonitrile, and it is important to use high-purity (HPLC grade) fresh solvents. The aqueous phase forms the strong eluent. Typically, this mobile phase is 100 mM ammonium formate, pH 4.5. (This mobile phase is MS-compatible, so you can use it in conjunction with an Agilent quadrupole time-of-flight MS). Columns are typically 2.1 × 150 mm, so you must optimize your instrument configuration to minimize dead volume. Similarly, you should remember that you can easily overload narrow-bore HILIC columns. Injection volumes of only 1 µL are common; larger volumes can lead to poor peak shape and loss of resolution.
 Enlarge
Enlarge
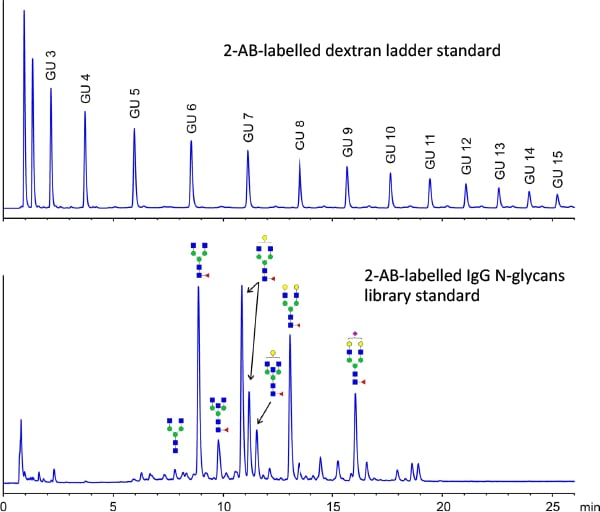
Figure 3. The Agilent AdvanceBio Glycan Mapping 2.7 µm, 2.1 × 150 mm column delivers fast, high-resolution separation of N-glycan labelled with 2-AB.
Optimized methods safeguard valuable samples
The Agilent dextran ladder and IgG N-glycan library standards help you optimize both sample preparation and column operating conditions before you analyze your valuable samples (Figure 3). You can use the retention times from the dextran ladder to determine the relative “glucose units” (GU values) for the peaks. You can then search online databases to determine possible glycan structures that correspond to these GU values.
High flow rates (0.5 mL/min on 2.1 mm id columns) are commonplace, but can potentially lead to high backpressures. Elevated operating temperatures (40, 50, or 60 °C) help to reduce eluent viscosity and therefore reduce backpressure and lead to improved peak shape. But you must use caution because temperature changes can alter selectivity of some N-glycans labelled with 2-AB.
Ultimately, you can select columns for faster separations when you require greater sample throughput, or run longer, shallower gradients when you need maximum resolution and separation of critical pairs of N-glycans.
Effective solutions for biologics and biosimilars
Biotherapeutics have enormous potential to improve human health. The number of approved protein and antibody therapeutics continues to grow as this important therapeutic class addresses unmet medical needs. At every stage in the process, from disease research to QA/QC and manufacturing, Agilent can help you make the right choices to move therapeutics successfully to market.
>> Update My Profile | Subscribe to Access Agilent | Article Directory