Agilent 101: An Introduction to Bio-Analytical Measurement
|
|

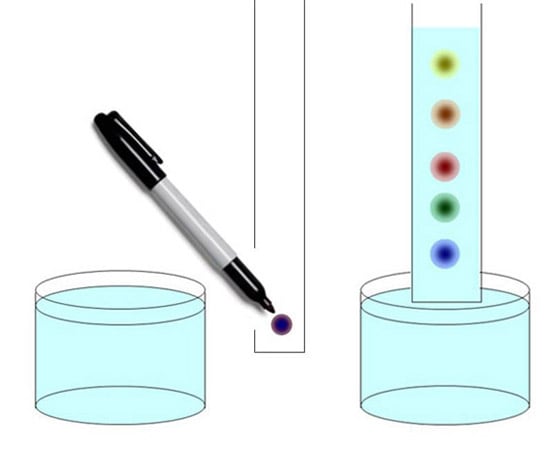
Our customers want to answer questions like, "What molecules are in there?" and "How much of each kind?" Sometimes, they want to know the shape and size of each molecule with questions like, "How many atoms of each atomic element?" and "How are the atoms connected to each other?" The best way to approach a complicated problem is to simplify it. Wouldn't it be great if we could measure each kind of molecule separately, without having to worry about all the others? That's what we call separation science, and Agilent is very good at it. So the first thing we try to do is to take this complicated mixture of molecules and separate it into each of its components. Then, once we've separated things out, we need techniques to detect, identify and quantify the amount of each component. There are lots of ways to do a molecular separation. If you have taken chemistry in school, you may recall precipitation. You put a chemical in your mixture that causes a chemical reaction. The stuff you're trying to measure falls to the bottom so you can weigh it. There's distillation, a time-honored technique that separates molecules based on boiling point. There's crystallization. If you take a mixture of chemicals and cool it down, some molecules will crystallize before others. There's electrophoresis, which is used in Agilent bioanalyzers, and selective hybridization, which is used in our DNA microarrays. Today I'm going to talk about gas chromatography and liquid chromatography because they're widely used in both our chemical analysis and life sciences instruments. Chromatography was invented in 1901, when Mikhail Tsvet discovered that he could separate chlorophyll, the green pigment in plants, into several colored bands. The name means literally "color writing." You can have some fun by doing the following paper chromatography experiment at home.
The column is kept in an oven to vaporize your sample, which might be a solid, liquid or gas at room temperature. You inject the sample into a flowing stream of some carrier gas, usually helium, to force the sample through the column. As the helium flows, some of the sample molecules move faster down the column than others, depending on how much they like to stick to the inside of the column. The different molecules will get separated. What you see in the end is a chromatogram, which is a plot of the quantity of molecules coming out of the column as a function of time.
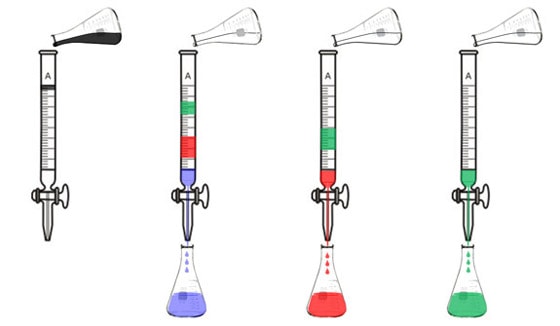
Liquid chromatography is similar. Originally, chemists used a glass tube that was filled with particles that molecules might stick to, like ground up chalk or sand, or carefully coated glass beads. They would pour their liquid sample into the tube and it would sit at the top of the column. Then they'd pour a solvent in and the different constituents would separate out. If they were analyzing a mixture of pigments, for example, the blue pigment might come out first, next the red one, and finally the green one.
This worked well, but it was rather laborious. Today, you can buy an Agilent HPLC system instead. HPLC means "high-performance liquid chromatography." Our HPLC is a marvel of German engineering. A system of two pumps produces very high pressure to force a liquid sample through a column. The two pumps enable you to use two different solvents, so you can gradually switch from one solvent composition to another. This is called gradient elution chromatography. The molecules that dissolve best in the first solvent will come out ahead of the ones that dissolve best in the second solvent.
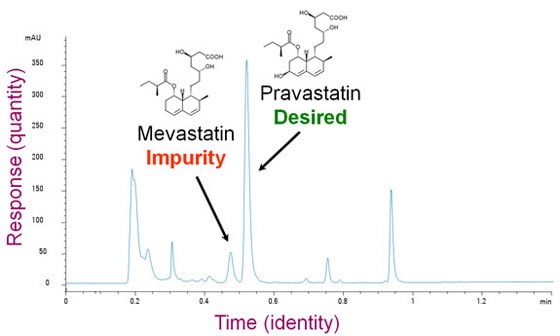
What would you use an HPLC for? Imagine that you work in a pharmaceutical company. Your job is to figure out a process to manufacture and purify a compound called pravastatin, which is good for treating high cholesterol. Unfortunately, your process also creates mevastatin, which is chemically very similar to pravastatin but might have unwanted side effects. You want to adjust the process to make only the desired molecule. You need a tool that can separate these things out and tell you how much there is of each. That's what liquid chromatography can do.
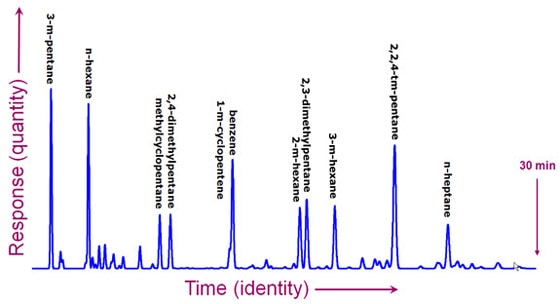
From separation to detectionSo now you've successfully separated a complex mixture into a stream of different molecules. In the case of paper chromatography, you could see the results. Usually, however, the chemicals are colorless and too dilute to see. You need a more sophisticated technique to detect them. There are many methods to choose from. Some are very specific and can tell you a lot about the molecule. Others can detect just about any molecule, but don't give much additional information. Imagine again that you're an Agilent customer. This time, you're a chemical engineer and you're responsible for the processes in an oil refinery that makes gasoline. You're worried about the octane rating and eliminating cancer-causing chemicals. There are molecules you want in the gasoline and others you don't want. Here is a chromatogram that took 30 minutes to produce in a gas chromatograph. It shows peaks for each of the different chemicals. The peaks were measured by a simple detector that can't tell the difference between molecules, but it can give you an approximate measure of the quantity of molecules in the gas as it exits the GC. It doesn't directly tell you which molecule accounts for each peak, but under the same conditions, a particular chemical will always show a peak at the same time.
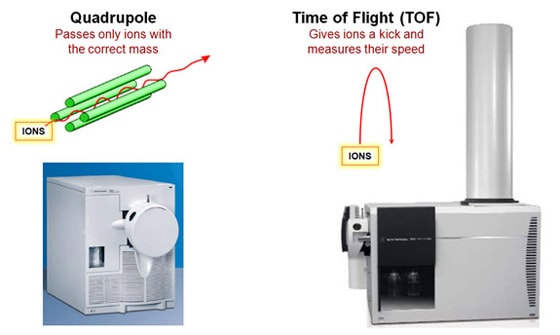
So how do you put labels on the peaks? One way is from having lots of experience in running test samples. But there is a better way, and Agilent would love to sell it to you! A mass spectrometer can tell you exactly what is the chemical composition of each peak. If you see a white box in someone’s lab that has a long vertical tube sticking out, it’s probably our time-of-flight mass spectrometer. If it’s a really long tube, it’s one of our high-resolution mass spectrometers. Agilent’s instruments use a reflectron in which the particles are shot upward and bounce off an electrostatic mirror at the top of the tube. That way, the tube can be half as long for a given resolution.
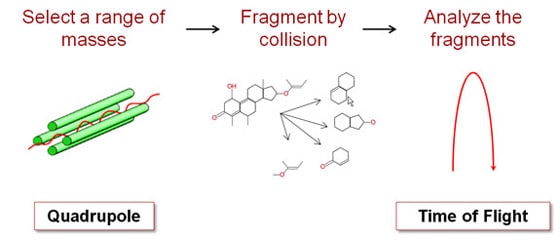
Mass spectroscopy is a powerful technique. It's very sensitive and extremely precise. And if one mass spectrometer is good, two must be even better, right? Right! We sell a lot of tandem mass spectrometers, with two separate mass selective stages. For example, we sell a QTOF, which means it's got a quadrupole front-end and a time-of-flight back-end. Why would you want two mass spectrometers in one instrument? What makes this powerful is what you do in between the two stages. Between the Q and the TOF you smash your original molecule into some other gas molecules, which breaks your molecule into fragments. Imagine that your quadrupole mass spectrometer told you that you have a particular organic compound with six carbon, 12 hydrogen and six oxygen atoms. You suspect it's a sugar, but it could be glucose, fructose or galactose. They all have the same chemical formula, but the atoms are connected differently. How do you figure out which one it is? If you're analyzing a larger molecule like a protein it gets worse, since the combinations are virtually endless. One way is to break the molecule into pieces and measure the mass of each piece. Different molecules will fragment in different ways. By analyzing the pieces, you can reconstruct what you started with. That's what we do with the QTOF. We use a quadrupole stage to select out just the molecule we care about. Then we bang it into the gas molecules to break it into pieces. Finally, we use the time-of-flight stage to tell us the mass of each fragment. We also sell a QQQ, or triple-quad, which has three quadrupole stages. The stage in the middle is where the collisions occur.
To review, first you separate your sample, usually by chromatography. Then you detect it with something like mass spectroscopy. In combination, these give you a really powerful chemical analysis method. Agilent pioneered the commercialization of products that combine chromatography and mass spectrometry in one instrument. GC-MS is gas chromatography followed by mass spectroscopy, while LC-MS is liquid chromatography followed by mass spectroscopy. As the molecules are coming out the end of the chromatograph, you send them directly into the mass spectrometer. You get a bunch of peaks that are measured to within a fraction of an atomic mass unit. With this kind of evidence, you can sort out the chemical composition in detail. We call combinations like GC-MS and LC-MS hyphenated techniques. Another example is LC-UV, in which liquid chromatography is combined with ultraviolet spectroscopy. Now that our new colleagues from Varian are on board, we can consider offering things like GC-FTIR (Fourier transform infrared) spectroscopy or LC-NMR (nuclear magnetic resonance) spectroscopy. But it gets even more complicated. We can do two-dimensional chromatography, in which we use two completely different chromatography methods on the same sample, one after the other. In one-dimensional chromatography, we might separate our molecules into 100 different parts. In two dimensions, it would be into 100 x 100, or 10,000 different parts. We can hyphenate that with mass spectroscopy or optical spectroscopy. Over the past decade, we’ve developed microfluidic techniques that enable our customers to do these analyses on very tiny samples, faster and more conveniently. One of our microfluidics products is the HPLC-Chip/MS. This stands for “high-performance liquid chromatography chip mass spectroscopy.” In this case, a plastic chip between the LC pumps and the mass spectrometer concentrates and separates the sample in very small volumes and performs the tricky task of putting an electric charge on large biomolecules and injecting them efficiently into a mass spectrometer. As you can see, separation and detection methods can become very sophisticated and complex. Agilent is especially strong in combining separation technologies with high-performance spectroscopy and detection techniques. This article has only skimmed the surface of our bio-analytical capabilities, leaving out things like our microarray technology, our electrophoresis products, our molecular biology and chemical reagents business, our automation technologies and our software tools. Thanks to our acquisition of Varian, we now have even more capabilities, including optical spectroscopy, nuclear magnetic resonance, X-ray crystallography, and vacuum products. Varian also extends our consumables business, which includes a large variety of separation columns for gas and liquid chromatography.
February 2011
|
||||||||||||||||||||||||||||||||||||||||||||